This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
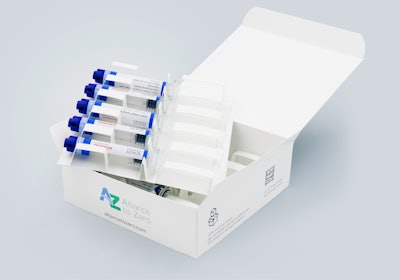
 The Cap-Lock Label from Schreiner MediPharm wraps around the syringe and cap, and irreversibly indicates first opening.
The Cap-Lock Label from Schreiner MediPharm wraps around the syringe and cap, and irreversibly indicates first opening.
The new solution features Cap-Lock, a functional label from Schreiner MediPharm that, in combination with the SCHOTT TOPPAC® infuse COC Syringe and a novel specialty cap, can completely eliminate the need for blister packaging. Due to a new cap design featuring the same diameter as the syringe barrel, Schreiner MediPharm’s Cap-Lock Label can be applied to the syringe in a reliable process. The functional label wraps around the syringe barrel and cap like a second skin, offering an irreversible first-opening indication that is automatically activated when the cap is opened. This approach ensures the integrity of the prefilled syringe from production to final use – discouraging tampering and misuse and allowing final users to immediately recognize if the syringe and label have been previously opened.
Aside from the first-opening indication, the Cap-Lock Label from Schreiner MediPharm performs additional protective functions traditionally associated with blister packaging and transfers them to the primary container; for example, a gas barrier and multi-level UV and light protection can be integrated into the label design. Extensive labeling space enables flexibility for customized branding, product information or drug color codes, and an RFID chip for automated unit-level tracking and digital first opening indication also can be integrated. Due to the combination of the COC syringe with a specialty cap and functional label with a sealing function, the filled syringes can be packaged in a volume-optimized top-load cardboard box consisting of 100 percent mono-material from Körber Pharma. This ensures optimized packaging and distribution processes in pharmaceutical production and requires substantially less space during transportation and storage.
Thus, the new packaging system not only offers considerable cost savings for pharmaceutical manufacturers but also makes a significant contribution to sustainability. In the case of the 5 ml SCHOTT TOPPAC® infuse, eliminating blisters allows an additional 1,260 syringes per pallet to be shipped, resulting in 16 containers saved per ten million syringes. In addition, the absence of blister packaging reduces plastic materials and plastic waste by 80 percent. For instance, during a one-way shipment of ten million 5 ml syringes from Hamburg to New York CO2 reductions of up to 87,400 kilograms can be achieved.
Consisting of the prefilled COC syringe, specialty cap, and Cap-Lock Label in combination with the cardboard box packaging, the system has already successfully passed ISTA 3A transportation testing. With this co-marketing solution, Schreiner MediPharm together with SCHOTT Pharma and Körber Pharma emphasizes its commitment to innovative and sustainable packaging solutions. The three partnering companies as founding members of the Alliance to Zero thus support pathways toward carbon footprint reduction throughout the value chain while ensuring user convenience and patient safety.






















