While brand owners may not think of consumers or patients as part of brand protection efforts, they may be a valuable piece of the puzzle. What if each and every customer was part of these efforts?
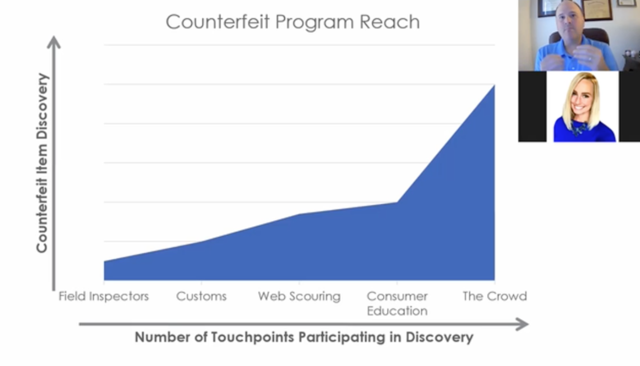
Systech recently presented its demo on the crowdsourcing concept for brand protection at PACK EXPO Connects in November. In this case, crowdsourcing is meant to compliment current anti-counterfeit efforts, such as the use of:
- corporate inspectors,
- customs searches, and
- web policing or IP enforcement.
But crowdsourcing is designed to have a wider reach than these other methods as it uses consumers to gather information, no longer relegated solely to efforts such as Wikipedia and GoFundMe.
With the increase in e-commerce, extra “boots on the ground” help.
“While online sales have consistently increased over the years, the current global pandemic has caused a significantly greater transition to online shopping, which means the industry is prime for counterfeiters looking to sell fake goods to unsuspecting consumers,” said Steve Tallant, senior director of market development at Systech in a recent blog post. “To make matters worse, it’s easy for counterfeit e-commerce sites to reopen under a different name after being shut down, making this a serious and systemic issue for all brands.”
How it works
e-Fingerprinting is a technology that creates a unique digital identifier for each and every product in the marketplace. Through a smartphone app, consumers can scan the UPC barcode on a product. The code’s e-fingerprint is then matched in the cloud, confirming to the consumer that they do indeed have an authentic item. The app can then drive the consumer to more information about the brand’s products, usage tips, and more.
Related article: Digital Brand Protection: Fingerprinting for Serialized and Non-Serialized Products
If the product is counterfeit, prompts on the app will direct the consumer to provide more information on the suspect item. Images of the item can also be attached to the consumer’s response. This information is then sent to corporate investigators.
For companies looking to crowdsource without alerting consumers, there are discreet ways of reaping the benefits. “Not everyone is comfortable being this transparent with their customers. The good news is, you don’t have to be,” Tallant explained in the post. “e-Fingerprinting enables Gamification, Super Shoppers and Connected Connoisseurs. All of these programs can be set up to send product authentication data discreetly back to you each time a user interacts with your product, or a counterfeit of your product.”
Counterfeiting and diversion result in $1.7 trillion lost annually according to the company’s findings. Online sales have increased 72% since June 2019, which unfortunately also provides more opportunity for counterfeit goods to be introduced into the legitimate supply chain.
“If we enable the crowd to authenticate every item in the field, we’re really bringing a huge power that will deter counterfeiters from going after your product because they’ll know that a critical mass of counterfeit products are being found and we’re shutting them down,” said Tallant at Systech’s PACK EXPO Connects demo (available here on-demand through March 31, 2021).
This system is intended to assure consumers that they are buying authentic, safe products. Potential benefits to brands include:
- Finding where counterfeits might be located and where legitimate merchandise is being authenticated
- Acquiring an inexpensive addition to anti-counterfeiting methods
- Dynamic interaction with consumers through the products they are purchasing
Crowdsourced data-driven insights include:
- Creation of heatmaps of illicit behavior or successful product sales
- Information and alerts for faster operational response times
- Strategic identification of supply chain gaps
- Trace maps of products’ journey, and lot and batch quality control
Related article: FDA Dec. Meeting: Drug Supply Chain Security Act Pilot Project Program