One group that stands poised to benefit from the evolution of the pharmaceutical and medical device industries are contract manufacturers (CMOs) and contract packagers (CPOs). As manufacturers strain to balance regulation compliance, expanding consumer demand, and shifting market realities, the option to contract out a portion of the production and packaging process becomes a viable strategy.
Currently, about one third of all processing and packaging in the pharmaceutical and medical device industries is handled by contractors. Manufacturers are utilizing contractors to shore up production shortages, run smaller batches that would slow down production in-house, and to manufacture products that require highly specialized equipment (such as blister packs and pre-filled syringes).
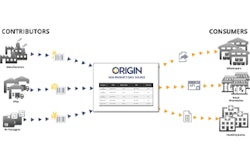
Occasionally a company will send all of their processing and packaging needs to a contractor, giving rise to the concept of “virtual manufacturing,” which allows companies to operate without investing in any physical production equipment.