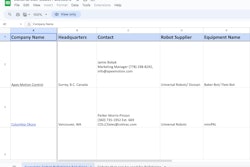
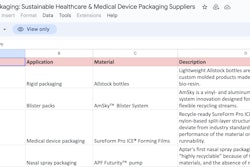
Genzyme had to “move all fill and finish for products destined for the U.S. out of its Allston, MA, plant by November 29,” reports in-Pharma Technologist.com.
That follows news that Genzyme would have to pay a $175 million fine and “cease some manufacturing operations at its facility in Allston,” said an earlier in-Pharma Technologist report. On May 25, its Web site noted “manufacturing and quality problems at the plant, which began with the discovery of viral contamination that forced Genzyme to temporarily halt production of several of its key products. “
“The new agreement replaces prior agreements between Genzyme and Hospira relating to filling and packaging of its drugs, including Cerezyme, Fabrazyme, Thyrogen and Myozyme,” said a report on Chicago Breaking Business.
According to the FDA, “Genzyme has agreed to a work plan for making facility improvements.”
Cambridge, MA-based Genzyme Corp. is a global biotech company. Hospira, Inc., Lake Forest, IL, is a global specialty pharmaceutical and medication delivery firm.
That follows news that Genzyme would have to pay a $175 million fine and “cease some manufacturing operations at its facility in Allston,” said an earlier in-Pharma Technologist report. On May 25, its Web site noted “manufacturing and quality problems at the plant, which began with the discovery of viral contamination that forced Genzyme to temporarily halt production of several of its key products. “
“The new agreement replaces prior agreements between Genzyme and Hospira relating to filling and packaging of its drugs, including Cerezyme, Fabrazyme, Thyrogen and Myozyme,” said a report on Chicago Breaking Business.
According to the FDA, “Genzyme has agreed to a work plan for making facility improvements.”
Cambridge, MA-based Genzyme Corp. is a global biotech company. Hospira, Inc., Lake Forest, IL, is a global specialty pharmaceutical and medication delivery firm.