In the following question-and-answer article, Healthcare Packaging (HCP) talks with Dr. Desmond G. Hunt, Ph.D., Senior Scientific Liaison, United States Pharmacopeial Convention (USP), about USP developments pertaining to temperature-sensitive packaging and how packaging professionals can get involved in the development of USP’s new general chapter <1083> on supply chain integrity.
HCP: Describe USP and its involvements.
Dr. Desmond G. Hunt: The United States Pharmacopeial Convention (USP) is a nonprofit scientific organization that develops standards for the identity, strength, quality, and purity of drugs and their ingredients, which are published in the United States Pharmacopeia and the National Formulary (USP–NF). USP–NF includes monographs, general chapters, and General Notices. A monograph is developed for a single article (e.g., drug substance, drug product, excipient), while a general chapter can apply across multiple articles. Above 1000 general chapters are intended to be informational, and contain no mandatory requirements unless specifically referenced in a monograph, General Notices, or a general chapter numbered below 1000. General chapters designated as below 1000 contain tests that are generally intended to be components of monographs for items recognized in USP or NF. Enforcement of applicable USP-NF standards is the responsibility of FDA and other government authorities in the U.S. and elsewhere; USP has no role in enforcement.
Healthcare Packaging: What developments should packaging professionals be aware of regarding US Pharmacopeia Guidance on temperature-controlled supply chain issues?
Hunt: In 2012, we introduced a new general chapter, <1083>, which deals with supply chain integrity. The goal for this chapter was to serve as a sister chapter to <1079> General Storage and
Distribution Practices for Drug Products. We had our workshop in May 2012, during which we hoped to gain input from stakeholders as to whether we were moving in the right direction by developing this new chapter.
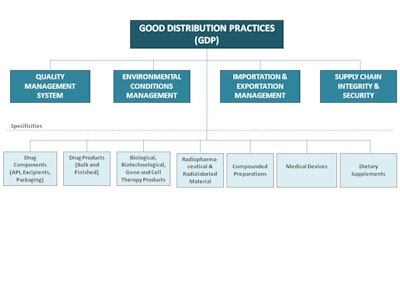
Two things that we heard loud and clear were (1) build off the current good distribution practices guidances and (2) develop a chapter(s) that addresses all USP products—from starting material to finished drugs. Taking that into consideration, we asked, “What are the four topics that are essential for a robust drug product supply chain?” The four areas that kept surfacing were: (1)
incorporation of good distribution practices in the organization’s quality management system; (2) environmental control management; (3) good importation and exportation practices and; (4) supply chain integrity and security.
Based on these four areas, USP has developed five different chapters, to date, that cover these topics. They haven’t been finalized yet, but ideally they would serve as a replacement for General Chapter <1079>. The new general chapters are scheduled to appear in Pharmacopeial Forum 40(2) [Mar.–Apr. 2014]. Pharmacopeial Forum (PF) is our bi-monthly journal in which USP proposes any revised or new standard for review. Nothing becomes finalized or official as a USP standard until it’s gone through a 90-day public comment in PF. At the end of that period, we take all the comments received and present them to our USP Expert Committee to review. The chapter is then revised accordingly. Once we are satisfied with the final document, we either move it to an official status or we can make changes to the content, which would then be republished in PF for further input.
HCP: Provide examples of specific packaging factors that will be considered.
Hunt: From the packaging perspective, you want to qualify your drug product packaging system to make sure it affords the right protection. It’s always important to focus on temperature, but we also try to highlight the importance of humidity because moisture permeating into a container-closure system can have an impact on drug product stability.
We do talk about technologies that are out there to monitor drug product temperature as a product moves through the supply chain. These technologies can be used to examine temperature and humidity variations over time and can be used to conduct temperature mapping of your facility and vehicles, or qualify a transportation packaging system. We know that excursions will happen.
When they do happen, how you address them and how you figure out whether they’re going to have an impact on the drug product are points we try to highlight.
HCP: Can our readers in packaging and logistics functions become involved in this USP guidance and can they help shape it?
Hunt: Absolutely! We can never have too many great minds or writers in a room. USP welcomes all interested stakeholders to participate in our standards-setting process. You can e-mail me at [email protected], or call me at 301.816.8341. If someone is interested and wants to be part of an effort, I am more than willing to put them to work. You can also go to (www.usp.org/usp-nf/pharmacopeial-forum). It’s a free online journal that requires one-time registration. We really want to encourage people from the packaging community to give us feedback.
HCP: Do USP and the U.S. Food and Drug Administration work together, and if so how?
Hunt: USP develops and revises standards, and FDA has the role of enforcing them. We try to find synergies between the two organizations because if USP goes in directions other than those of interest to FDA, it will not be beneficial to those affected by the standards. We try to work together on every USP Expert Panel and Committee, and we try to have FDA liaisons take part in our discussions. We get feedback from them on various topics.