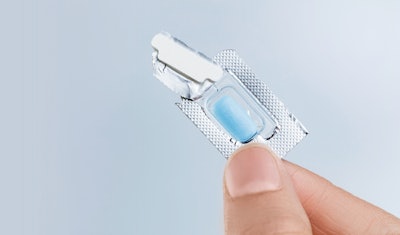
Aptar CSP Technologies has announced a new manufacturing site able to produce its Activ-Blister™ solutions for oral slide dose drugs in Europe. The move is part of a key strategic effort to expand production of Activ-Blister technology globally and is the result of a three-way collaboration between Aptar CSP, Uhlmann (a leading pharmaceutical packaging equipment manufacturer), and Ivers-Lee (a local contract manufacturing organization).
The collaboration consists of a fully automated Uhlmann blister machine validated to package oral solid dose drug products with Activ-Blister solutions heat-staked to the foil at a rate of up to 250 blisters per min. The equipment is designed to integrate Activ-Blister technology into the blister cavity with precision placement. The equipment has been fully validated and is in place at Ivers-Lee, a CMO capable of manufacturing Activ-Blister solutions from R&D to commercialization. A CMO in EMEA will support regional customers and ensure speed to market.
“Expanding global manufacturing sites for Activ-Blister technology reflects our commitment to delivering global access to innovative active material science solutions for patients and consumers,” says Badre Hammond, VP commercial operations and GM APAC, Aptar CSP Technologies. “With manufacturing capabilities now available in both the U.S. and EMEA, we will turn our attention to bringing production of this technology to the Asia market.”
Activ-Blister solutions integrate Aptar CSP’s proprietary three-phase Activ-Polymer™ platform technology into individual blister cavities to provide microclimate protection for sensitive drug products. This technology can be customized specifically for the drug developer’s formulation to provide a broad spectrum of specific drug protection, including moisture adsorption, and oxygen and odor scavenging. The technology can also scavenge volatile organic compounds (VOCs) and emit aromas.
























