In our top 10 of 2019, Dirk Rogers highlights news on the particles front (article here), as FDA expects a visual inspection program and parenteral manufacturers should expect questions during inspections.
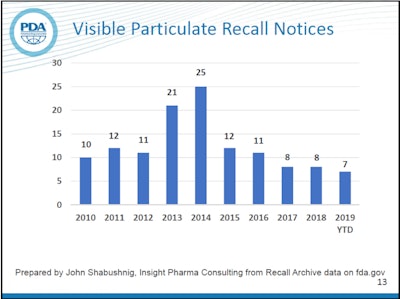
Visual inspection of parenteral drug packaging remains a priority for manufacturers and the FDA, as explained by John Shabushnig, PhD of Insight Pharma Consulting and Richard Watson of Merck at the 2019 Parenteral Drug Association (PDA)/FDA Join Regulatory Conference held in Washington D.C. in Sep. 2019. Shabushnig monitors FDA recall notices, filtering on those that are due to visible particulate in parenterals.





















