This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
Mettler-Toledo Hi-Speed will host a webinar to identify and explain the questions you need to ask when considering a potential serialization equipment supplier. A wealth of information will be provided on current serialization technology and technical components and we will explain how these can be integrated into a production line without affecting performance, availability or quality.
The most important factors you need to take into consideration when choosing marking, verification and checkweighing equipment for a serialization program:
• Fulfilling E-pedigree, Track & Trace and Serialization legal requirements
• The Serialization Equipment Challenge - 5 major challenges facing all pharmaceutical companies
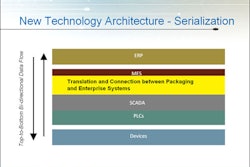
• Individual Serialization Components - Printers, vision systems, RIFD and precision product handling
• Serialization Systems Solutions
• Asking the Right Questions - How to recognize a good offering including a detailed checklist
TIMES:
• Wednesday, July 14, 2010 2:00 PM EDT
• Wednesday, August 18, 2010 2:00 PM EDT
To register, please visit www.mt.com/hs-serializationwebinar, or email [email protected] and request to be registered. You will receive an email reminder with log-in details for the Webinar.