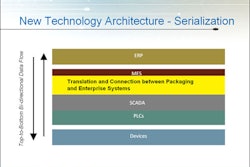
Securing Pharma reported May 21 that Brazil “may back away from its current plans for implementing a national serialization system for medicines and adopt one closer to the model [that] has just gone live in Turkey.”
Recently, Healthcare Packaging interviewed Cognex executives who noted, “Turkey took a leading role in addressing fraud in the reimbursement process by mandating new drug traceability processes. These processes also help control the proliferation of counterfeit drugs.”
Meanwhile, in the U.S., the U Connect 2010 Conference will include a June 8, “Preparing for Serialization and Visibility within the U.S. Pharmaceutical Supply Chain, 2015 Readiness Workshop” event, which will provide GS1 insights into pharmaceutical supply chain, serialization, and Pedigree.
-Jim Butschli, Editor