This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
Emerson, an industrial technology expert delivering advanced automation solutions, is empowering manufacturers with seamless data mobility across a new suite of life sciences software in the DeltaV Automation Platform. Providing key automation solutions from the earliest stages of recipe development through commercial manufacturing, Emerson’s suite of life sciences software makes it easier for organizations to select and connect critical tools necessary to bring quality treatments to patients faster, safer and more sustainably.
The traditional process of creating a new treatment from research through clinical trials to commercial manufacturing is largely manual and intensely time consuming. Life sciences companies spend substantial resources in both time and money performing the steps necessary to move information across various applications to successfully develop and manufacture new products. With a wide variety of technology solutions from different providers in the various segments of the business, data can quickly become siloed in individual applications, making it difficult to move from stage to stage, and increasing the likelihood of error.
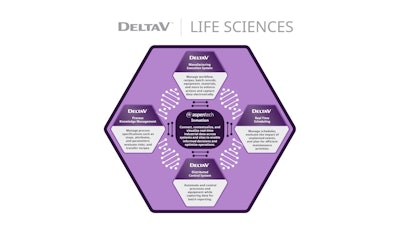
To overcome these challenges, Emerson’s life sciences software suite is purpose-built within the comprehensive DeltaV Automation Platform, streamlining the connection of critical components across the development and commercialization pipeline to create a more collaborative environment. For example, users can create a general recipe and risk profile in DeltaV Process and Knowledge Management (PKM), and then, using the DeltaV Tech Transfer Hub—a sophisticated mapping tool created through Emerson’s One-Click Technology Transfer initiative—quickly send the pertinent recipe information to the DeltaV Manufacturing Execution System (MES) or the DeltaV Distributed Control System (DCS).
“Easier integration of critical technologies in the treatment development pipeline is key to unlocking the five value drivers of life sciences: pipeline acceleration, flexible manufacturing, operational integrity, real-time release, and sustainable operations,” said Nathan Pettus, president of Emerson’s process systems and solutions business. “Combining the suite of life sciences software solutions under the unified DeltaV Automation Platform enables life sciences organizations to embrace the Boundless Automation vision of seamless data mobility. Ultimately, with this vision, organizations can unlock flexibility and operational excellence capabilities that are crucial to delivering next-generation treatments to patients in need.”
Leveraging a common data fabric made possible by Emerson’s AspenTech Inmation real-time data platform, the software suite including DeltaV PKM, DeltaV DCS, DeltaV MES, and DeltaV Real Time Scheduling can be integrated more easily, reducing the need for customization.






















