This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
Dickson developed this quick reference guide to help users integrate Dickson's FDA-compliant data loggers and chart recorders into full-compliance best practices.
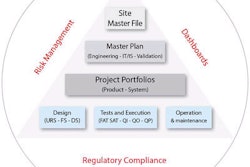
Chris Sorensen, Dickson VP Sales and Marketing explains, “Dicksonware™ Secure is extremely easy-to-use software that enables FDA-regulated food and drug processors to adhere to the safety standards that have been developed to ensure consumer and patient safety. A guiding principle of all Dickson R&D efforts is that the temperature, humidity, pressure and electronic signal monitoring instrumentation that we release to the market is unsurpassed in terms of ease-of-use. Creating this FDA 21 CFR Part 11 Compliance checklist is an extension of this core value and provides an easy to understand guide for choosing between the secure version of Dicksonware or the more generic version that is not designed for regulatory compliance. It's our job to make our customers' jobs as easy as possible when it comes to environmental monitoring and regulatory compliance. Today, when so many products that the FDA monitors come from abroad it is equally important to ensure that customers worldwide understand what U.S. regulations mandate.”
Chris Sorensen, Dickson VP Sales and Marketing explains, “Dicksonware™ Secure is extremely easy-to-use software that enables FDA-regulated food and drug processors to adhere to the safety standards that have been developed to ensure consumer and patient safety. A guiding principle of all Dickson R&D efforts is that the temperature, humidity, pressure and electronic signal monitoring instrumentation that we release to the market is unsurpassed in terms of ease-of-use. Creating this FDA 21 CFR Part 11 Compliance checklist is an extension of this core value and provides an easy to understand guide for choosing between the secure version of Dicksonware or the more generic version that is not designed for regulatory compliance. It's our job to make our customers' jobs as easy as possible when it comes to environmental monitoring and regulatory compliance. Today, when so many products that the FDA monitors come from abroad it is equally important to ensure that customers worldwide understand what U.S. regulations mandate.”
Companies in this press-release