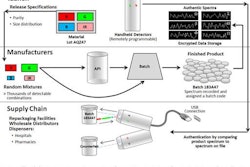
The FDA recalled the TigerPaw II by Maquet Medical Systems, because incomplete closure of the it may result in tissue tears and/or bleeding, including a possible tear on the left atrial wall during use of the device.
Maquet Medical Systems received 51 reports of adverse events and one death, according to the FDA.
The TigerPaw II is a surgical staple used to close tissue in the left atrial appendage of the heart.
The affected devices were distributed April 1, 2013 through March 23, 2015.