When primetime television dramas such as Hawaii Five-o and CSI: Cyber air episodes about the world of counterfeit and stolen prescription pharmaceuticals and unscrupulous Internet medication sales, you sense these issues are becoming more mainstream.
Pharmaceutical manufacturers and packaging suppliers have worked for decades to develop processes and materials to combat counterfeits, striving to remain a step ahead of the criminals.
In this article, Andrew S. Janoff, Ph.D., and Founder and CEO of Taaneh, Inc., reports on research concerning the development of diamond as an authentication tool to combat counterfeit pharmaceuticals…
In the pharmaceutical industry, sales of counterfeit drugs now total an estimated $350 billion each year. In addition to lost revenue to manufacturers, falsified medicines put millions of people at risk because these products often have suboptimal efficacy and safety profiles. A key consideration in authentication technologies is the ability to tag and trace both product and packaging at any stage in the supply chain from manufacturer to consumer, even when a drug is separated from its packaging. Technologies must also be incorporated into packaging protocols with minimal investment and impact on production timelines.
In 2013, the FDA mandated that over the next 10 years companies must implement an interoperable system that can more accurately identify and trace drugs. To meet this goal, industry will need to identify more advanced options relatively soon. Many current technologies can help to trace drugs or packaging, but not both—and are useless if drugs are separated from packaging. In addition, many options require significant changes in manufacturing and packaging that can make their implementation unviable.
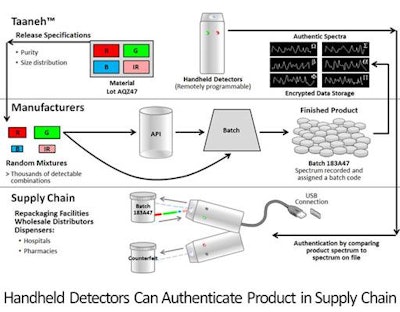
Recently, leaders in drug development and product packaging have been considering the use of diamond particles to support the new levels of authentication outlined by the FDA. Diamond is an allotrope of carbon, which is on the FDA’s inactive ingredient list. When exposed to light, diamond particles can emit an almost unlimited range of distinct spectral signatures that are impossible to replicate. Diamond powder can be readily added to drug formulations and to packaging and ink, making it possible to verify authenticity easily even when drugs are not in their original packaging. Authenticity can be confirmed at any point of the distribution chain from manufacturing to consumer.
With the addition of diamond particles to drug and packaging, verification can be accomplished via remotely programmable handheld detection devices. Spectra recorded from the finished product, uniquely identifying its lot and expiration date, can be sent to, and maintained in, a secure database. Using handheld detectors that can be programmed through a secure network, the spectra of the product are compared to the spectra assigned to the product lot in the database, providing a precise confirmation in real time.
But the use of diamond in product authentication also represents an important potential advantage related to packaging. With diamond, packaging can be printed with ink containing the same random mixture of diamond that is added to the drug product. As a result, manufacturers can authenticate drug and packaging independently or together. The use of diamond also supports confirmation of authenticity even when drug and packaging are separated.
Taaneh is currently developing the use of diamond as an authentication tool, with promising results. For drug packaging professionals concerned with both oral and infusion-based dosage forms, the use of diamond represents a new way to provide the levels of authentication speed and accuracy that the new regulations require. Diamond can be quickly and easily incorporated into current manufacturing and printing protocols, and unlike unique indicia or bar codes, it requires no overt changes in package design or production facility retooling.