Sonoco ThermoSafe, a unit of Sonoco and a global provider of temperature assurance packaging, launched a bulk temperature-controlled solution to optimize air shipments of temperature-sensitive biologics and pharmaceutical products for durations exceeding five days.
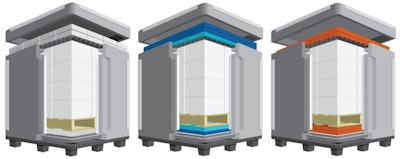
The new Quarter PMC pallet shipper platform maximizes the space on an LD7 PMC air pallet used in wide-body aircrafts. The platform includes solutions for 2° to 8°C, 15° to 25°C and frozen temperature ranges. Each solution has a universal pack-out design to be used in both hot and cold seasons while also accommodating the demands of cross-hemispheric shipments.
Products feature patent-pending technologies such as interlocking L-shaped panels and eliminate corner leaks and reduce edge leaks.
Also, ConvecTECHTM dispersion panels maximize natural convection to eliminate side refrigerants, increasing payload and simplifying packing procedures.
Following the Sonoco i6 Innovation Process, the Quarter PMC was one of ThermoSafe’s first products to be developed through its disciplined step-by-step process, which combines insights, ideation and invention. With performance and simplicity in design, these products pique the interest in users of both passive and active solutions.
The Quarter PMC Pallet Shipper will be available in both U.S. and European facilities.