Billions of medical devices are sterilized with ethylene oxide (EtO) each year. While EtO has made headlines and caused facility closures for unsafe environmental exposure and resulting health risks, for many years, there was no readily available process to serve as an alternative for medical device sterilization.
In 2019, the FDA set up innovation challenges to identify new sterilization methods and reduce EtO emissions. With limited facilities and significant device volumes, EtO sterilization providers become impacted leading to long lead times for sterilization.
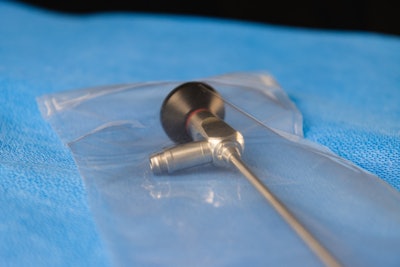
At the[PACK]out this week, Megan Frost, PhD, and Kurt Yockey, both of Sterile State LLC, unveiled a new technology for medical device sterilization.
Yockey has worked with hospitals, nurses, medical device companies, and the Joint Commission is his tenure. “I'm here to introduce that our system is really very simple. Devices are placed into an enclosure, it's sealed, and then shipped immediately to customers. It's that simple,” he said. “There are no regional sterilization facilities, no sterilizer fees, no logistics, no delays, no waiting for sterilization, and it's completely safe.”
This technology, nitric oxide (NO) inside a polymer, was announced May 9. If successful, it could help assuage industry fears about having sufficient sterile medical devices while reducing risks to environments and worker safety.
Dr. Frost, CTO at Sterile State LLC, has worked on technology for 20 years, perfecting the sterilant and delivery system: “I started out using this technology to make implanted medical devices. The reason I'm telling you that is because it's a really safe technology, and it was developed to be at a standard much safer than what we used for packaging devices that aren't actually going to be implanted within the human body.”
Continue to the full article here.