10 at 11:00 am MST for medical device manufacturers entitled, Regulatory Considerations for Managing Changes in Packaging Materials and Test Methods for Validation of Sterile Barrier Materials.
The webinar will provide information to medical device manufacturers on (1) how to assess the impact a change in materials or processes may have on old or new packaging and (2) help them to know if they need to revalidate packaging following any changes to the materials or processes deemed at risk.
“It is very important medical device manufacturers respond to triggers built into their change management process as it relates to material changes for their packaging,” said Sherri Robbins, Nelson Laboratories' director of regulatory affairs. “We're providing information and tools that will help them make those decisions.”
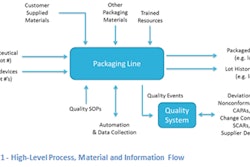
Medical device and drug manufacturers need to consider the regulatory requirements associated in managing changes for packaging materials or critical supplies as well as preparing for and performing any revalidation that may be required.
Regulatory Considerations for process or material change (More information):
• Detect and document the change.
• Assess risk and impact of product change.
• Mitigation, acceptance verification and validation.
• Dissemination of change information to personnel, customers and regulatory authorities.
• Change management documentation and approvals.
Once manufacturers complete regulatory considerations, they may find they need to reassess their packaging processes or materials through three test categories to validate packaging:
• Integrity test - Comprised of the bubble emission test and dye migration test.
• Strength test - Comprised of the seal peel test and burst test.
• Microbial Barrier test - Comprised of the F1608 microbial ranking and the whole package aerosol challenge.
Manufacturers need to put into place the triggers to start the management process resulting from changes with materials or supplies used in packaging. Regulatory agencies including FDA expect manufacturers to conduct their due diligence regarding any changes to their packaging.
Nelson Laboratories provides consulting and testing services to help medical device manufacturers manage their change management process.
The webinar will provide information to medical device manufacturers on (1) how to assess the impact a change in materials or processes may have on old or new packaging and (2) help them to know if they need to revalidate packaging following any changes to the materials or processes deemed at risk.
“It is very important medical device manufacturers respond to triggers built into their change management process as it relates to material changes for their packaging,” said Sherri Robbins, Nelson Laboratories' director of regulatory affairs. “We're providing information and tools that will help them make those decisions.”
Medical device and drug manufacturers need to consider the regulatory requirements associated in managing changes for packaging materials or critical supplies as well as preparing for and performing any revalidation that may be required.
Regulatory Considerations for process or material change (More information):
• Detect and document the change.
• Assess risk and impact of product change.
• Mitigation, acceptance verification and validation.
• Dissemination of change information to personnel, customers and regulatory authorities.
• Change management documentation and approvals.
Once manufacturers complete regulatory considerations, they may find they need to reassess their packaging processes or materials through three test categories to validate packaging:
• Integrity test - Comprised of the bubble emission test and dye migration test.
• Strength test - Comprised of the seal peel test and burst test.
• Microbial Barrier test - Comprised of the F1608 microbial ranking and the whole package aerosol challenge.
Manufacturers need to put into place the triggers to start the management process resulting from changes with materials or supplies used in packaging. Regulatory agencies including FDA expect manufacturers to conduct their due diligence regarding any changes to their packaging.
Nelson Laboratories provides consulting and testing services to help medical device manufacturers manage their change management process.
Companies in this press-release