The European Medicines Verification Organisation (EMVO) is taking a balanced approach to translating the EU Falsified Medicines Directive into practice. The non-profit is focused on advancing the European Medicines Verification System (EMVS), for the purpose of medicine verification and the enhancement of patient safety, as Andreas Walter, General Manager of the EMVO, explained at PDA's 2016 Pharmaceutical Cold and Supply Chain Logistics Conference on Oct. 12, 2016.
The EMVO welcomes not only pharmaceutical manufacturers, but hospitals, wholesalers and pharmacies. Andreas Walter noted, “Each actor has its place,” and that all members have one vote, which keeps everyone on the same level.
The organization has built the European Hub, to which national systems, pharmaceutical manufacturers and parallel distributors can connect with directly. In creating a system that nations and companies can access, the EMVO expects the EU Hub will:
-
Ensure interoperability between national systems
-
Secure cross-border trade and handling of multi-country packs
-
Provide cost savings for connecting manufacturers
-
Support the establishment of standard interfaces
Companies that wish to onboard must pay a fee and go through a rigorous onboarding process (both contractual and technical) with legitimacy checks. Because manufacturers will upload critical product data, the legitimacy of any party requesting access to the Hub must be established through a meticulous process.
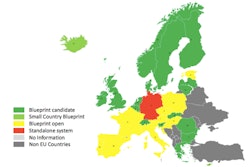
The country that each company is based in must also onboard, through the creation of a National Medicines Verification Organization (NMVO) that will establish a National System to connect to the European Hub. A hallmark of the EMVO’s program is its blueprint approach for National Systems, so that the wheel isn’t reinvented 32 times (or more).
Often a source of concern, Walter was clear that the EMVO believes the data belongs to those who create it, and that members should pay a participation fee to the system governance costs in order to vote. This means that stakeholders who want to be full members should pay for part of the NMVO. “This is the difference between affiliate membership and full membership,” he said.
Many countries are taking up this offer. In Walter’s eyes, the blueprint standard is the only approach that will enable countries to be ready in the two years left to meet EU-FMD requirements. “It’s the only way to achieve the timeline,” he said.
Unfortunately, many manufacturers are lagging behind in their EU-FMD efforts, and it is a great source of concern for Walter. Overall, the EMVO has onboarded approximately 38 manufacturers out of an estimated 2500 in the EU, with 15 of those currently in the test environment and five in the production environment, sending data via the Hub to the existing German system. (The German system is a standalone system, as it was created before the blueprint system in 2012.)
Manufacturers across the globe who must meet EU-FMD requirements are urged to reach out to the EMVO out as soon as possible. It may take six months to implement the verification system, and up to one year for testing.
Though onboarding may be a rigorous process, Walter explained that the benefits are worth the effort. The EMVO seeks to create cost-savings for manufacturers in establishing one single point of data entry for all of Europe versus a decentralized approach with 32 systems. Beyond software, the EMVO offers to outsource some services to get NMVOs lean and streamlined.
The organization also fosters a collaborative and transparent environment with constant dialogue, which will ultimately help all partners involved as they face and overcome new challenges. Walter noted, “It’s a cost effective, harmonized way to translate regulation to compliance.”