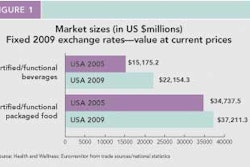
As healthcare continues to evolve with an eye to expenses, patients are becoming more responsible for their own health outcomes. In this scenario, functional packaging must communicate with patients and better enable them to comply with medication regimens. No surprise then that compliance-prompting packaging is a focus for Rockford, IL-based contract packager Anderson Packaging, Inc..
Anderson, one of three companies within the AmerisourceBergen Packaging Group, along with American Health Packaging and Brecon Pharmaceuticals, opened its eighth facility in Rockford in March.
“We've seen continuous growth over the years and have been expanding our business,” notes Justin Schroeder, director, marketing and business development for Anderson Packaging.
Much of Anderson's growth, he points out, “comes from compliance-prompting packaging from both generic and branded manufacturers.” What's generating that business? “I think compliance packaging can help make sure you are taking your medication when you are supposed to in order to achieve the end result of better health.”
Better patient compliance often involves unit-dose packaging. Schroeder notes, “We've seen increased support of unit-dose compliance packaging for oral solids, as well as growth in parenterals, in particular with biologics growth. We've done several things in our business to support that. One would be opening the eighth facility here in Rockford, which is a specialty pharmaceutical center designed to support potent compounds.” By that, he's referring to products requiring site segregation, for example hormonal products, cytotoxins, and other potent compounds.
Biologics value presents challenges
The new 29,000-sq-ft facility for packaging specialty/biologic products is a Class 100,000 ISO 8-equivalent clean room environment, “designed under the guidance of Safe-Bridge Consultants [www.safebridge.com], which is a global industry standards organization that assists in terms of rating potent compounds and determining how you should handle them,” notes Schroeder.
He identifies these products as oral solids, transdermals, and parenterals, such as those used for cancer drugs/oncology products. “We did a significant expansion of our refrigerated storage to support those types of products because often they need cold chain refrigeration,” he says.
“These products need special handling for refrigeration and material handling,” he adds. “There's a need to keep scrap levels at a minimum. We run some products here that cost up to a $1,000 per unit. Compare that to a generic aspirin, for example, that's fractions of pennies per unit.” Some of these biologics are lyophilized, and some are liquids.
Overall, Anderson Packaging's eight facilities provide more than 1 million sq-ft of manufacturing space for pharmaceutical and specialized packaging services. Manufacturers send product to Anderson for packaging, with the CP shipping packaged product back to those manufacturers for sale to pharmacies, hospitals, and long-term healthcare providers.
Six of Anderson's facilities focus on pharmaceutical contract packaging, while two support healthcare-related offerings such as oral care, beauty, and other products.
Driving innovation
Economic factors in some cases have resulted in personnel cutbacks at manufacturing facilities, limiting the ability of these companies to engineer innovation into their packaging. That adds responsibility and opportunity for contract packagers and packaging suppliers.
Anderson continues to drive innovation with its emphasis on child-resistant/senior-friendly pharmaceutical packaging. Schroeder notes, “As an organization, we had traditionally been independent regarding any specific package type. We supported whatever our customer wanted us to package because we were focused more on automation and making the compliance package cost-effective for manufacturers.
“But customers kept coming back to us asking, 'What else is out there, what else is new?' They felt there was not much innovation in the marketplace. So, that's when Anderson and our AmerisourceBergen Packaging Group started to get involved in terms of designing our own proprietary packaging.”
Clinical trials involvement
Looking ahead, Schroeder says, “Anderson is becoming more active in clinical packaging, storage, distribution, and related services. Traditionally we have focused on commercial applications. Ten to 15 years ago we had an active clinical packaging business, and now we are redeveloping that position.”
The move requires flexibility on Anderson's part in that clinical trials projects are smaller. “We may run 50 or 500 packs for a clinical trial, whereas a commercial project may run two million packs,” says Schroeder. “And there's a great level of accountability, making sure that each and every packaged product is perfect and meets the stringent requirements for blinding. We have also started construction on a standalone clinical packaging, storage, and distribution facility that is scheduled to open in October of this year.
“We help companies design not only the clinical trial packages, but we also support them as they move into the commercialization stage, where we assist with sales and distribution efforts through the AmerisourceBergen Specialty Group companies, including third-party distribution.”
Anderson, one of three companies within the AmerisourceBergen Packaging Group, along with American Health Packaging and Brecon Pharmaceuticals, opened its eighth facility in Rockford in March.
“We've seen continuous growth over the years and have been expanding our business,” notes Justin Schroeder, director, marketing and business development for Anderson Packaging.
Much of Anderson's growth, he points out, “comes from compliance-prompting packaging from both generic and branded manufacturers.” What's generating that business? “I think compliance packaging can help make sure you are taking your medication when you are supposed to in order to achieve the end result of better health.”
Better patient compliance often involves unit-dose packaging. Schroeder notes, “We've seen increased support of unit-dose compliance packaging for oral solids, as well as growth in parenterals, in particular with biologics growth. We've done several things in our business to support that. One would be opening the eighth facility here in Rockford, which is a specialty pharmaceutical center designed to support potent compounds.” By that, he's referring to products requiring site segregation, for example hormonal products, cytotoxins, and other potent compounds.
Biologics value presents challenges
The new 29,000-sq-ft facility for packaging specialty/biologic products is a Class 100,000 ISO 8-equivalent clean room environment, “designed under the guidance of Safe-Bridge Consultants [www.safebridge.com], which is a global industry standards organization that assists in terms of rating potent compounds and determining how you should handle them,” notes Schroeder.
He identifies these products as oral solids, transdermals, and parenterals, such as those used for cancer drugs/oncology products. “We did a significant expansion of our refrigerated storage to support those types of products because often they need cold chain refrigeration,” he says.
“These products need special handling for refrigeration and material handling,” he adds. “There's a need to keep scrap levels at a minimum. We run some products here that cost up to a $1,000 per unit. Compare that to a generic aspirin, for example, that's fractions of pennies per unit.” Some of these biologics are lyophilized, and some are liquids.
Overall, Anderson Packaging's eight facilities provide more than 1 million sq-ft of manufacturing space for pharmaceutical and specialized packaging services. Manufacturers send product to Anderson for packaging, with the CP shipping packaged product back to those manufacturers for sale to pharmacies, hospitals, and long-term healthcare providers.
Six of Anderson's facilities focus on pharmaceutical contract packaging, while two support healthcare-related offerings such as oral care, beauty, and other products.
Driving innovation
Economic factors in some cases have resulted in personnel cutbacks at manufacturing facilities, limiting the ability of these companies to engineer innovation into their packaging. That adds responsibility and opportunity for contract packagers and packaging suppliers.
Anderson continues to drive innovation with its emphasis on child-resistant/senior-friendly pharmaceutical packaging. Schroeder notes, “As an organization, we had traditionally been independent regarding any specific package type. We supported whatever our customer wanted us to package because we were focused more on automation and making the compliance package cost-effective for manufacturers.
“But customers kept coming back to us asking, 'What else is out there, what else is new?' They felt there was not much innovation in the marketplace. So, that's when Anderson and our AmerisourceBergen Packaging Group started to get involved in terms of designing our own proprietary packaging.”
Clinical trials involvement
Looking ahead, Schroeder says, “Anderson is becoming more active in clinical packaging, storage, distribution, and related services. Traditionally we have focused on commercial applications. Ten to 15 years ago we had an active clinical packaging business, and now we are redeveloping that position.”
The move requires flexibility on Anderson's part in that clinical trials projects are smaller. “We may run 50 or 500 packs for a clinical trial, whereas a commercial project may run two million packs,” says Schroeder. “And there's a great level of accountability, making sure that each and every packaged product is perfect and meets the stringent requirements for blinding. We have also started construction on a standalone clinical packaging, storage, and distribution facility that is scheduled to open in October of this year.
“We help companies design not only the clinical trial packages, but we also support them as they move into the commercialization stage, where we assist with sales and distribution efforts through the AmerisourceBergen Specialty Group companies, including third-party distribution.”