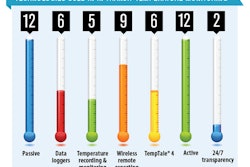
Qualifying active and passive shippers for global use, global harmonization or standardization, and customs’ handling of temperature-sensitive shipments are among the primary challenges concerning temperature-sensitive product packaging and logistics professionals.
So say respondents to an exclusive Healthcare Packaging survey conducted electronically in early September.
Asked about the most irritating matter in keeping up with legislation, regulatory requirements, and government compliance issues pertaining to temperature-sensitive shipments, one survey respondent noted, “Legislation proclaims ‘how’ rather than being results-oriented, so it encumbers the process and discourages innovation.”
In this article, Healthcare Packaging presents many of the most thoughtful verbatim responses in a Q&A-style format, with questions noted in boldface type. Some responses were edited for clarity.
HCP: What is your primary challenge concerning temperature-sensitive product packaging and logistics?
“Compliance to the qualification and validation of the storage and transportation facilities in two seasons.” “Extreme distances and extreme temperatures.”
“The most important challenge is in logistics and transportation of 2° to 8°C products, since even in air shipments it is very difficult to ensure temperature compliance.”
“Avoiding temperature spikes and documenting the spikes that do occur.”
“The main thing is trying to manage the logistics involved with qualifying active and passive shippers for global use. Another big challenge centers around having the design qualification and operational qualification performed to my company’s ambient temperature profiles.”
“Ensuring [that] qualifications mirror real-world usage as closely as possible. Not only through the use of appropriate thermal profiles, but [also] by utilizing appropriate placebo product and pack-out conditions.”
“Finding qualified people to correctly activate the monitoring devices as well as retrieve the data.”
“Lack of infrastructure in various countries.”
“Shippers ignoring our storage requirements and maintaining control during shipping when shipments are out of our control.”
“Customs’ ability to clear temperature-sensitive shipments quickly—or provide correct and adequate storage to maintain required temperature.”
“Finding people that really understand the importance of a robust packaging solution and assess all risks involved in the cool chain logistics process.”
HCP: What is your single biggest irritation when it comes to keeping up with legislation, regulatory requirements, and government compliance issues?
“The most irritating regulation is having trucks standing at the border for clearance for hours [where] sometimes temperature excursions occur due to opening and checking of goods.”
“Wading through excessive verbiage…nonspecific language…not having time to sort through the verbiage.”
“Lack of knowledge from people in charge of compliance issues.”
“The combination of monitoring multiple countries without harmonization or a world standard.”
“The largest irritation comes when dealing with multiple staggered global launches that have drastically varied requirements. Additionally, the reluctance of some regulatory bodies to accept technical assessments or testing performed in laboratory settings to fulfill cold chain distribution requirements can result in excessive amounts of extremely expensive full-scale distribution qualifications.”
“Regulations without industry input.”
“Multiple sources without a clear-cut and definitive authority/concise place to go for updates and information.”
“Frequently changing legislation” or “Keeping up with changing requirements.”
“Legislation that proclaims ‘how’ rather than being results-oriented, so it encumbers the process and discourages innovation.”
“Customers not understanding regulatory compliance.“
“No one seems to have a true understanding of what is involved in monitoring needs for the FDA.”
HCP: What advice would you share with a new team member?
“Always double-check paperwork and confirm.”
“You can never be overly cautious when it comes to ‘packaging’ and ‘shipping' validation.”
“Follow protocol and do it with an experienced co-worker.”
“When a problem is discovered, handle it immediately and set deadlines for answers.”
“It is best to have multiple vendor contacts to consult with and maintain communication so you are up-to-date on the latest and greatest.”
“Do not speculate.”
“Learn from experience and then come up with new perspectives and ideas.”
“Management should fully support continuous training and orientation for the quality control team including standards and guidelines.”
“Focus on new technology that guarantees excellent quality, reliability, safety, identity, product shelf-life claims, stability, wholesomeness, and overall presentation of the product as well as productivity and low cost.”
HCP: What references does your team find most beneficial?
“I would recommend looking at the ISTA and FDA regulations to help understand the expectations from these regulatory or industry bodies. I would also provide references to supplier solutions with temperature-sensitive shipment and packaging.”
“Talk to all vendors [and] educate yourself as much as possible.”