From FDA’s Sickle Cell Disease awareness efforts to an award-winning inhalation pack to Xavier’s new graduate program, there is a lot going on when it comes to clinical trials.
Healthcare Packaging has put together short briefs about some of the most interesting developments happening in this area.
FDA efforts
The U.S. FDA is developing educational materials to raise awareness about clinical trials for Sickle Cell Disease. Some of these materials include a webinar for patients to learn how to find clinical trials for SCD and meetings with SCD stakeholder groups to determine the best strategies to raise awareness about clinical trials participation. More information is available at hcpgo.to/281.
Co-packaging generics
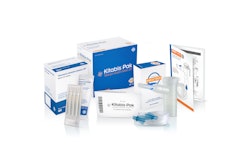
PARI Respiratory Equipment, a worldwide developer and manufacturer of aerosol delivery systems, won an American Package Design Award for the K• Kitabis Pak®, which was approved by the FDA last December.
Kitabis Pak is reportedly represents the first co-packaging of generic
tobramycin inhalation solution with a PARI LC PLUS(®) Nebulizer for patients with cystic fibrosis.
"This award highlights all the hard work we put into this unique product of nebulized drug and device,” says Jan Zimmermann, Portfolio Manager for Kitabis Pak. “In creating a package that is easy for patients to use, we also wanted to convey that the components suit each other. By using complimentary colors, the packaging visually reflects the fact that tobramycin inhalation solution was designed to be delivered by the PARI LC PLUS Nebulizer."
"Not only is the packaging attractive, it ensures that with every prescription, every patient has access to the only FDA-approved nebulizer handset for tobramycin inhalation solution and the same nebulizer used in clinical trials. It is gratifying that the creative package for this important combination of therapy and delivery device puts us alongside other design winners, such as Target, Burt's Bees, Revlon, and Tory Burch," says Ashley Weigand, Director of Marketing for PARI Respiratory Equipment.
Sharp expansions
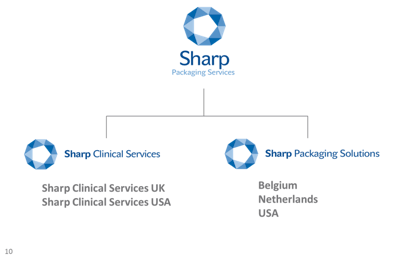
Sharp Clinical Services expanded its storage and packaging facilities in the U.K. by an additional 5,000 sq-feet. A new facility based in Crickhowell is now the fourth unit of the existing Waller House building and comprises of 150 additional pallet spaces and two assembly lines. The company’s investment is in response to new product introductions approved by regulatory authorities. Sharp Clinical Services provides specialist clinical supply chain services to both large pharmaceutical and small biotech companies in the U.K. and U.S., from drug product development, analytical, and manufacturing services through to increasingly complex clinical trial supplies packaging, clinical labeling, and clinical distribution services.
Expanding facilities
Catalent, a global provider of advanced delivery technologies and development products for drugs, biologics, and consumer health products, announced that it has expanded its Singapore facility, which recently received GMP approval from the Health Science Authority, to provide secondary packaging and labeling capabilities. The site is approved for GMP across all its activities, including secondary packaging, label printing, storage, distribution, returns, and destruction management. The Singapore facility is said to be a strategic hub for Catalent’s Asia Pacific clinical supply network, with expertise in storage and distribution of clinical supplies.
Instant verification
The Association of Clinical Research Professionals (ACRP) announced a new way for employers, hiring managers, and others in the clinical research community to instantly verify the competency of clinical trial personnel.
ACRP and its affiliate, the Academy of Clinical Research Professionals, have partnered with ProExam Vault to introduce digital badging technology to the clinical research community.
New degree program
Xavier University announced a new graduate degree for 2015 through a program that will be part of its Health Services Administration and is a partnership between Xavier and CTI, Clinical Trials, Inc., a Cincinnati company. The Master of Science in Health Economic and Clinical Outcomes Research (HECOR) is a 40 credit hour professional program offering students a foundation in health economics and health services research. Visit hcpgo.to/282 for more details.
Embedded barcodes
At press time, Microscan was demonstrating embedded barcode readers and machine vision inspection cameras designed to meet precise requirements for the form, fit, and function for the life of clinical instruments at the 2015 AACC Clinical Lab Expo.
NIH
The National Institutes of Health (NIH) in late June announced a clinical trial investigating a potential treatment for alcohol use disorder (AUD). The study will assess the safety and efficacy of gabapentin enacarbil (HORIZANT) in extended-release tablets for treating moderate to severe AUD. NIAAA is part of the National Institutes of Health.
Development center
Advantar Labs, a contract provider of pharmaceutical product development services, announced the opening of a non-sterile manufacturing facility. The completion of the new manufacturing space provides offerings ranging from development through clinical manufacture for low-to-medium-volume niche non-sterile products.