Medical devices can be complex assemblies and include intricate shapes and delicate parts, all of which makes cleaning during manufacturing particularly difficult. Despite those challenges, the requirement for perfect cleaning before packaging is unequivocal: complete removal of particulate, oils or inorganic contaminants introduced during the manufacturing processes is critical. Additionally, it is vital the parts be perfectly dry to avoid any pyrogenic activity.
Vapor degreasing is re-emerging as a popular option in the medical device industry, especially in relation to replacement of older aqueous cleaning systems. This technology delivers the highest level of cleanliness to ensure patient safety and product performance and it generally does so at the lowest possible costs.
A few decades ago, the technology was the preferred method of cleaning medical devices because of its ease of use and reliable cleaning performance. However, in the late 1990s, environmental concerns fueled an industry-wide trend to switch from this process—which at that time used chemicals harmful to the ozone—to aqueous-based cleaning systems. Although there were disadvantages to using the aqueous cleaning systems, the environmental issues linked to many cleaning solvents at that time outweighed the benefits of vapor degreasing.
But aqueous cleaning has important considerations. The typical aqueous setup will likely be larger than a vapor degreaser of equal capacity; have complex high-pressure spray systems or mechanical agitation systems, and large heaters for the detergent baths. When dealing with delicate parts such as those in medical devices, the functionality of an aqueous system can be limited.
Recent advances in solvent technology have generated environmentally sound, low-temperature cleaning options that also minimize bioburden issues, which have led to renewed interest in cleaning through vapor degreasing.
The process
The process of vapor degreasing not only ensures the cleanliness of the device, but also satisfies the economic and regulatory requirements needed in medical component manufacture.
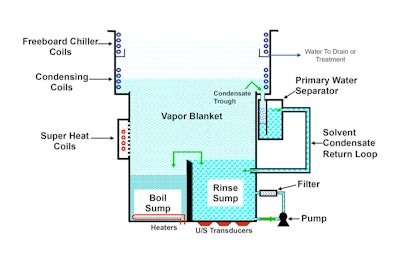
A typical vapor degreasing system consist of two chambers, both filled with solvent. In one chamber, the solvent is heated to a boil, which then generates a vapor cloud that rises to meet cooling coils. These cooling coils cause the vapors to condense and return to their liquid state. The system then channels this liquid back to the second chamber, the rinse chamber.
The dirty parts go through the vapors and into the boil sump for their first cleaning. Then the parts move into the rinse sump. The rinse sump always contains pure, clean solvent distilled from the solvent vapors.
In an apples-to-apples comparison, solvents clean more effectively than water because of inherent chemical characteristics of the fluid. The high density, high Kb values, low viscosity and low surface tension of solvents, combined with their volatility, allow them to clean effectively. They dry quickly and completely, leaving no residues on parts after they exit the vapor degreaser. In recent tests for biocompatibility with major medical device makers, solvent systems have not failed in removal of contaminants.
Solvents cleaning vs. water-based systems
Instinctively, water seems like it would be the more environmentally friendly option; water is cheap and “green.”
Bacteria grow in water. In contrast, solvents are inherently hostile to the growth of bacteria, which helps to manage bioburden issues in the manufacturing process, especially when components or finished devices get into packages immediately after cleaning. Even trace amounts of moisture can facilitate the growth of bacteria and create bioburden issues. This may compromise the ability to properly sterilize the device and requiring re-cleaning of the device or disposal of it altogether.
With aqueous systems, relatively complex process controls and water treatment systems must be used to ensure the water used does not complicate bioburden issues. Waste-water treatment systems are large, complex, and use large quantities of electricity.
Additionally, aqueous rinsing and drying processes are much more complex than vapor degreasing. Water-systems use energy-hungry blowers, air knives and heated dryers to dry hard-to-reach nooks and crannies. Even then, spotting or corrosion of parts can be a problem if not carefully managed. In contrast, vapor degreasers deliver clean, dry parts at room temperature without those large and noisy subsystems.
Aqueous cleaners also introduce large amounts of water into the manufacturing space. This leads to increased energy costs, as the building environmental systems must remove that humidity from the atmosphere.
In short, vapor degreasers offer a process that is more chemically effective at removing contaminants than aqueous systems. They do not require additional mechanical action or increased temperatures to be effective.
The hidden costs of aqueous cleaning
Managers should carefully consider all the hidden costs of aqueous cleaning:
-
Aqueous cleaning systems generate a wastewater stream that requires treatment before discharge. Water discharge directly to sewer systems or surface waters is, almost universally, problematic.
-
Aqueous cleaning has difficulty cleaning in tight spaces or tiny parts. Managers can resolve this problem by increasing the number and pressure of water jet sprays. This abusive cleaning process can damage delicate components and fine finishes.
-
Aqueous systems also require high temperatures to be effective, while solvents have lower boiling points — near room temperature. This means vapor degreasers use far less electrical energy.
-
Parts must also be dry after aqueous cleaning, requiring still more energy. The process controls required to eliminate bioburden issues add significantly to the complexity and costs of the cleaning process.
It is important to consider all of the environmental, operational and patient safety factors when selecting a method of cleaning components in the medical device industry.