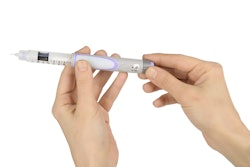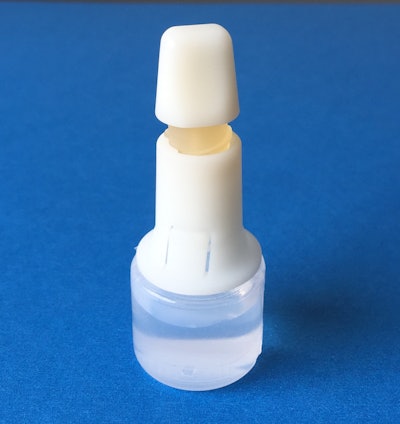
HealthStar, Inc., a reseller and rebuilder of Blow/Fill/Seal machinery, announces the MD-DropperCap™, a new dropper bottle technology for BFS applications.
This patent-pending design produces metered-dose eye drops with standard BFS technology, without the added step of isolation/insertion technology.
In the past, it has been necessary to employ isolation/insertion technology to achieve a controlled drop delivery for ophthalmic-type products. In this legacy process, a pre-assembled dropper and cap are inserted into the bottle after container formation, prior to seal mold closure. Production rates are negatively impacted (as much as a 50% reduction in output), since this process results in long molding cycle times of 25 to 28 seconds. In addition, the process requires the use of an isolator, which nearly doubles the cost of the machinery. Validation and machine operation are also much more complicated as a result.
MD-DropperCap eliminates the need for isolation/insertion technology. Containers are molded using standard BFS manufacturing machines and procedures. Cycle times are maintained at normal BFS output levels (typically about 12 seconds), improving productivity. The pre-assembled and sterilized dropper caps are snapped onto the BFS containers (post-manufacturing in a clean environment). The contents of the bottle are never in contact with the MD-DropperCap until activated by the patient. The result is a low-cost, easy to manufacture, safe container closure system for controlled drop delivery applications.
BFS has long been recognized as an advanced aseptic manufacturing technique. The company says the use of the new MD-DropperCap for BFS applications competes favorably with conventional container filling, tip insertion and capping processes.
For conventional processes, regulatory guidance dictates the handling of pre-sterilized bottles, tips and caps under cleanroom conditions, resulting in a RABS (Remote Access Barrier System) requirement for new ophthalmic manufacturing operations. Comparing the initial cost of facilities, machinery and day to day operations, the new MD-DropperCap proves BFS technology to be less expensive and easier to use than traditional manufacturing techniques.
Prototype samples are available and manufacturing quantities can be produced as required according to customer specifications.