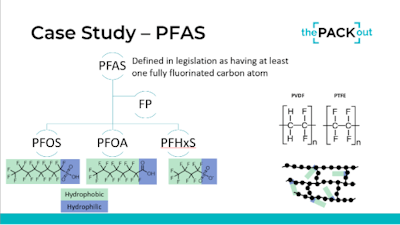
While the healthcare packaging and medical device communities have been exempted from certain material regulations, recent discussions have highlighted the need for companies to prepare for changing laws, especially those pertaining to per- and polyfluoroalkyl substances (PFAS), commonly referred to as "forever chemicals." These chemicals do not readily breakdown in the body or environment and have been linked in studies to endocrine disruption, certain cancers, and more.
During a session at the[PACK]out in May 2023, experts from Boston Scientific and Amcor shared insights on the global regulatory landscape and its implications for healthcare brand owners. To start, Alex Bowman, R&D manager at Amcor, emphasized the importance of staying informed and prepared for regulatory changes that will impact the life science packaging community, underscoring the need for companies to anticipate and adapt to these shifts.