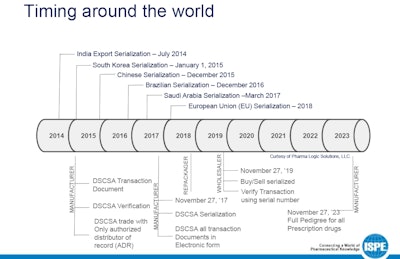
Even with serialization deadlines approaching, many manufacturers are still confused about the regulatory requirements of the Drug Supply Chain Security Act (DSCSA) and supporting technologies. Among packagers that do understand the idea and motives behind the law, practical experience is lacking.
As managing partner at Pharma Logic Solutions, LLC, William Fletcher is helping to shed light on serialization trends and approaches. He’s aided numerous life science and biologics companies on their road to serialization by developing their strategies, requirements and vendor selection projects.
Planning issues
It’s no secret that investing time in the planning stages will make for a smoother implementation process; a clear strategy, roadmap or charter is one of the keys to success. Ultimately, the project should be run internally, and not by a solution provider.
“One piece of advice I give is to engage all areas, from IT and packaging down to distribution in the process early,” Fletcher says. By working out your designs and process stages in advance of implementation, you can get through some of the challenges. He explains, “You don’t want to go through the expense of implementing and putting symbols on the product, only to find out that you have some issues when you get down to your shipping area.”
Often overlooked, plan for validation from the start. Fletcher advises that companies make sure requirements are measurable, “and that the requirements can transcend down to functional specifications.” Then ensure that the requirements in functional specs can be translated all the way down to be validated against.
Among the lessons learned, he cites that packaging changes are not considered soon enough. Understand that there will be label and artwork changes along the way to incorporate artwork while making room for the datamatrix code (For more on labeling, see Encoding and Labeling Issues, below).
Vendor selection
Define your requirements independent of commercial systems, and consider compartmentalizing solutions—the all-encompassing control option isn’t necessarily the best for you. Fletcher says, “Some projects I see are too focused on a particular solution. They find out as they move forward that other solutions are actually better suited for their business.” Additionally, ensure that you are defining governance over service providers. “Rather than having them tell you, you tell them what your requirements are,” he says, and make sure they can provide what you need, particularly in terms of exchanging information about allocated serial numbers and receiving back the serialized hierarchy.
Take time to evaluate suppliers early on, particularly for enterprise serial number management systems. Fletcher has seen companies have knee-jerk decisions and select systems without going through the user requirement process. “Now they’re trying to validate a system—they have no user requirements, nothing to validate against,” he laments. In some cases, they engage Fletcher to develop materials after the fact that they should have had ahead of time. If the solution is provided in a hosted environment, there are additional validation complications: another party performs the installation and operational qualifications, without the regulatory group vetting those processes, and they may have to go through the performance qualification without any training materials.
Visible helper codes
Companies use helper codes to help aggregate products together in packaging, but visible helper codes can also cause problems down the road. Fletcher says he’s recently seen studies in which large pharma companies sent samples of encoded products into the supply chain and discovered that people were scanning helper codes and believing that the product was misencoded. One study found that 30% of the recipients in the supply chain thought the product was not properly encoded because they scanned the first symbol they saw (the helper code) on the bottom of the bottle, instead of looking on the side for the proper code.
Failing to accommodate global requirements
Many companies are scrambling to add serialized lines, so it’s understandable that some choose to focus on the minimum U.S. requirements to meet looming deadlines. But that may be a mistake in terms of global commerce. Take aggregation for example: Fletcher sees a lot of companies that have skipped over aggregation because it’s not required in the U.S., but he points out, “It’s something that you’ll end up tackling anyway.” Beyond global requirements, he highly recommends aggregation to support suspect product investigations.
According to Fletcher, there is no reason not to qualify your system to support regulations from around the globe. “Some systems out there don’t, and that promise to do this in the future. You may want to take a different look and make sure your selection process includes all of these.”
Encoding and labeling issues
It’s also very important to consider how products move through the supply chain. Now that there are regulations requiring that people scan product labels, situations are coming up that have never posed a problem in the past. Often, labeling on the cases has not been examined in a long time, and there may be inconsistencies between labels across products within the same facility.
Fletcher says he sees the use of outdated GS1 encoding including Application Identifier 22, which was sunsetted in 2013. He encourages companies to harmonize labeling and to bring labeling in compliance of GS1.
People are relying on scanning these labels to pick up the lot information, and in some countries, the serialization. “If they can’t read that label or the label is not properly encoded, the most likely scenario is that those products will be returned to you as mislabeled or unusable,” Fletcher explains. “And that can be very expensive both in terms of processing and in terms of the gap it puts in your supply chain. The missing product in the supply chain must be replenished.” Considering these issues ahead of time can help you avoid time-consuming reactive situations later on as you fill the supply chain with newly encoded products.
Beyond unreadable labels, Fletcher also sees:
• Improperly encoded products that have a larger datamatrix code than necessary.
• Situations in which cartons are designed without considering that tamper-evident seals will be placed over the flap of a carton. Companies may become wrapped up in designing packaging, only to realize in testing that the tamper-evident seal is covering the datamatrix symbol. There are, however, ways you can structure your cartons so that they don’t need tamper-evident seals. Fletcher notes, “There is a flap model that, once the product’s inside, it can’t be opened without tearing the cardboard.” By using this technique, you eliminate the seal and you are able to use the full panel for encoded information.
Current trends
With his extensive experience in serialization and track-and-trace projects, Fletcher is in a unique position to see the following emerging trends across the industry, which he presented recently at the Pharma Expo (www.pharmaexpo.com) Conference in Las Vegas:
• Some pharmaceutical manufacturers with established serialized lines are offering contract packaging services to get more use out of their lines and boost their ROI. This can be a worthwhile option to remain compliant if you are still working on implementing serialization in your facility.
• Pre-coding of labels and cartons is becoming a popular technique in North America. It’s widely popular in other countries, especially in India and throughout the Pacific Rim. Several large life science companies have been pre-printing their labels and pre-encoding their cartons for years. This practice makes it easy for them to utilize their equipment and reduces their setup time in the room, and also allows them to deal with any issues as labels or cartons are being printed. This helps avoid lost-time, where workers are standing around in the packaging line waiting for problems to be solved.
• Kiosks for case and pallet aggregation.
• As mentioned above, eliminating visible helper codes used for aggregation to avoid supply chain confusion.
• Compartmentalizing packaging operations to reduce complexity and add flexibility.
• Eliminating site-based servers to reduce risk. Many of the newer systems are capable of communicating directly with the enterprise systems. Some are dependent on site-based systems, which can become a single point of failure. Fletcher says, “A lot of those site-based systems were developed so that once you purchased them, you’d be obligated to buy your line systems from that vendor.”
• Use of Electronic Data Interchange (EDI) Advance Shipping Notices (ASN) for transaction document (TD). Though a lot of companies are not capable of supporting EDI, it is something Fletcher advises looking into, as well as how you utilize it to gain visibility into operations.
• Establishment of Electronic Product Code Information Services (EPCIS) for collaboration. Fletcher is a proponent of EPCIS, but is candid about the topic. It has many communication and automation benefits, but he is not sure of how widely accepted it will be, as manufacturers may opt to continue using EDI to meet requirements.