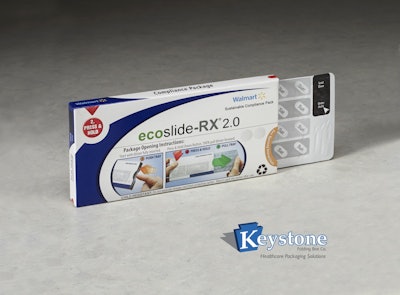
The new Ecoslide-RX 2.0 from Keystone Folding Box Co. enhances user-friendliness, including redesigned lock/unlock functionality and enhanced graphics, providing more dynamic user instructions.
The package passed Consumer Product Safety Commission (CPSC) protocol testing for child resistance and once again received the highest rating, F=1. In addition to demonstrating a high level of senior friendliness, Ecoslide RX 2.0 was also reported by patients to be “easy to open” in consumer testing.
Walmart plans to incorporate the next-generation compliance package into its pharmacies nationwide beginning in 2015. Previously, Walmart had helped to further drive the use of patient-adherence calendarized packaging in the U.S. with its Dec. 2012 launch of paperboard-based Ecoslide-RX packs supplied by Keystone Folding Box Co.
“With Ecoslide RX 2.0, the overall consumer experience has been improved: the opening procedure of the new package is more intuitive, and minimal effort is required to unlock the package. Enhanced graphics present operating instructions that are easily understood—a benefit particularly to seniors who, understandably, comprise a significant percentage of prescription medication users,” says Ward Smith, Director of Marketing at Keystone Folding Box Company. “Major pharmaceutical manufacturers are expressing strong interest in this package based on the overall customer experience.”
Compliance packaging helps prompt patients to take medications correctly. Many compliance packages offer day-of-the-week calendarization for each individual dose, which has been shown to significantly increase patients’ likelihood to adhere to medication regimens. Keystone’s latest package design is reported to be more environmentally sustainable than competing packages, hence its Ecoslide moniker.
In 2011, a published peer-reviewed study of more than 300,000 Walmart pharmacy patients during a two-year period concluded that compliance/ adherence packaging improved adherence and persistency. The same Walmart study showed a significant ROI for the incremental cost of the compliance package over traditional bottles.
Ecoslide-RX 2.0 works for prescription products, physicians’ samples, and clinical trial materials. It allows for large, easy-to-read type on all sides conducive to clear, simple opening instructions, and offers a reliable locking mechanism. The compliance pack also is easy to stack in medicine cabinets and convenient to transport. Blister packages have less risk of contamination from dropping, spilling or moisture, and can be equipped with weekly calendars/reminders that help consumers keep track of their medications.
Keystone Folding Box Co. manufactures and designs paperboard packaging, also serving as a design center and source for non-paperboard packaging components