Neffy
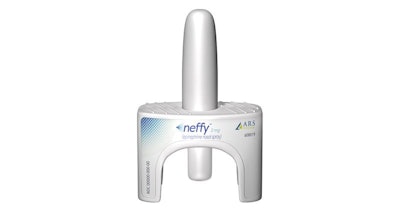
According to a recent CBS News article, the FDA’s outside advisers voted in favor of a needle-free epinephrine nasal spray product called Neffy, that could soon be the first of its kind to treat severe allergic reactions. The nasal spray is designed to deliver a 2mg dose of epinephrine capable of reversing life-threatening symptoms of an allergic reaction. The company is seeking FDA approval for anyone weighing over 30 kg (~66 lbs).