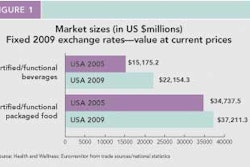
This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
The MedWatch May 2010 Drug Safety Labeling Changes posting includes 23 products with safety labeling changes to the following sections: BOXED WARNING, CONTRAINDICATIONS, WARNINGS, PRECAUTIONS, ADVERSE REACTIONS, PATIENT PACKAGE INSERT, and MEDICATION GUIDE.
The "Summary Page" provides a listing of drug names and safety labeling sections revised:
http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm214902.htm
Clicking on a drug product name in the Summary View will take you to the "detailed view" page, which identifies safety labeling sections and subsections revised, along with a brief summary of new or modified safety information.
The following drugs had modifications to the CONTRAINDICATIONS and WARNINGS sections:
Camptosar (irinotecan HCl)
Cervidil (dinoprostone)
Droxia and Hydrea (hydroxyurea)
NovoLog Mix 70/30 (70% insulin aspart protamine suspension and 30% insulin aspart [rDNA origin])
Parnate (tranylcypromine sulfate)
PrandiMet (repaglinide/metformin HCl fixed-dose combination)
Remeron (mirtazapine) tablets and RemeronSolTab (mirtazapine)
Selzentry (maraviroc)
Tekturna HCT (aliskiren/hydrochlorothiazide)
Vaprisol (conivaptan)
Zyprexa (olanzapine)
The "Summary Page" provides a listing of drug names and safety labeling sections revised:
http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm214902.htm
Clicking on a drug product name in the Summary View will take you to the "detailed view" page, which identifies safety labeling sections and subsections revised, along with a brief summary of new or modified safety information.
The following drugs had modifications to the CONTRAINDICATIONS and WARNINGS sections:
Camptosar (irinotecan HCl)
Cervidil (dinoprostone)
Droxia and Hydrea (hydroxyurea)
NovoLog Mix 70/30 (70% insulin aspart protamine suspension and 30% insulin aspart [rDNA origin])
Parnate (tranylcypromine sulfate)
PrandiMet (repaglinide/metformin HCl fixed-dose combination)
Remeron (mirtazapine) tablets and RemeronSolTab (mirtazapine)
Selzentry (maraviroc)
Tekturna HCT (aliskiren/hydrochlorothiazide)
Vaprisol (conivaptan)
Zyprexa (olanzapine)