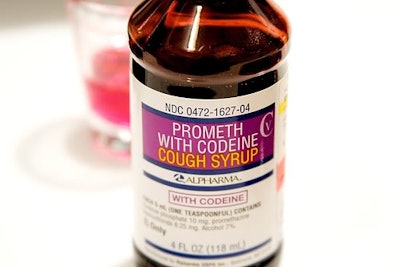
An FDA MedWatch article posted on January 11th said that the risks of prescription opioid cough and cold medicines outweigh the benefits. Thus, the FDA is requiring the addition of safety information about the risks of misuse, abuse, addiction, overdose, death, and slowed or difficult breathing to the warnings on the boxes. The FDA is also limiting the use of these medicines to people 18 years or older.
The FDA is also considering enforcing regulations on OTC codeine cough medicines in states where it is legal. The Safety Alert notes that health care professionals should be aware of the updated age range for these products, and parents and caregivers should avoid use on children.






















