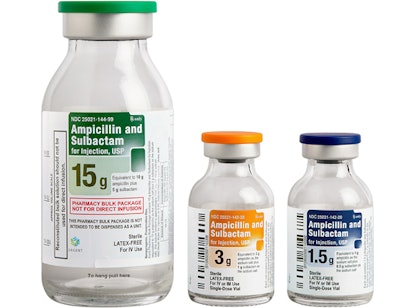
Ampicillin and Sulbactam / Image: AuroMedics
A recent Safety Alert for Human Medical Products from the FDA noted the recall of vials due to the presence of glass particles. The voluntary recall, which is relevant at the hospital level, concerns AuroMedics Pharma’s Ampicillin and Sulbactam for Injection USP in single-dose vials. Just one lot consisting of 10 vials was affected.
The presence of glass particulate in an intravenous drug can cause blockage and clotting in blood vessels, which may be life-threatening. Auromedics has notified distributors and customers via letter and is currently arranging for the return and replacement of all affected product.