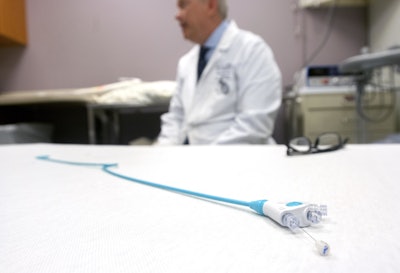
According to an article from JournalStar, a doctor is seeking FDA approval for his medical device that could erase the need for 300,000 procedures annually. ConvertX, invented by Dr. Bob Smouse, is a device used during urinary tract operations. When a stone or scar tissue obstructs the ureter, a condition most common in cancer patients, the surgical repair consists of two procedures. “In one surgery, doctors insert a catheter, which we'll call 'Device A' that drains urine from the kidney to an external bag; the catheter is removed a few days later, when doctors place a stent, 'Device B,' in the blocked urethra to allow urine to drain past the blockage in a second surgery,” writes JournalStar ‘s Laura Nightengale.
ConvertX marries technologies to convert from Device A to Device B in a matter of seconds, eliminating the need for a second invasive procedure. The device spares the patient's time and money as well as exposure to radiation. Dr. Smouse is the founder of California-based BrighWater Medical, the company that developed ConvertX. BrightWater recently closed a $5.7 million Series A financing round led by OSF Ventures, the investment arm of OSF HealthCare.





















