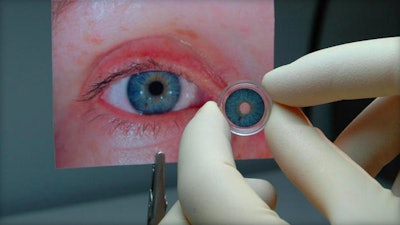
A recent CBS News article contained good news for anyone with a damaged iris in their eye: the FDA has just approved the first artificial iris. The iris is responsible for regulating how much light enters the eye, and those with compromised irises often suffer from light sensitivity and other vision problems. The CustomFlex Artificial Iris can be surgically inserted into the eye to correct the issues and improve the appearance of the eye.
The artificial iris is composed of thin, foldable medical-grade silicon that’s custom-fitted and colored for each individual patient. The device was deemed safe and effective after a study found that it decreased light sensitivity and improved health-related quality of life in 70% of participants.






















