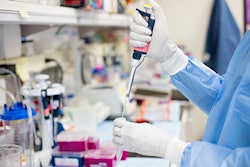
The ReShape Integrated Dual Balloon System, also known as ReShape Dual Balloon, was approved by the FDA as a new medical device to help treat obesity.
The ReShape Dual Balloon device is delivered into the stomach via the mouth through a minimally invasive endoscopic procedure. The outpatient procedure usually takes less than 30 minutes while a patient is under mild sedation. Once in place, the balloon device is inflated with a sterile solution, which takes up room in the stomach.
“This new balloon device provides doctors and patients with a new non-surgical option that can be quickly implanted, is non-permanent, and can be easily removed," said William Maisel, M.D., M.P.H., acting director of the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health.
It is meant to be temporary and should be removed six months after it is inserted.