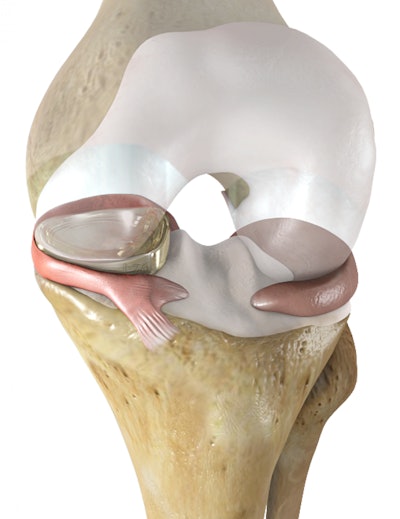
NUsurface Meniscus Implant / Image: Active Implants
According to a recent MDDI article, people suffering from meniscus surgery will likely have a new treatment option. The FDA has declared Active Implants’ NUsurface meniscus implant a breakthrough device. The artificial meniscus is currently available in Europe, but still needs clearance by the FDA to hit the market in the United States. The FDA’s breakthrough devices program was established to speed up the development and review process for medical devices that are novel or offer new technology for patients with “life-threatening or irreversibly debilitating conditions.”