Comprehensive serialization efforts are in full swing on both sides of the Atlantic. Even with the FDA’s recent issuance of a one-year delay in enforcement of the U.S. Drug Supply Chain Security Act to Nov. 26, 2018, the DSCSA law does not change.
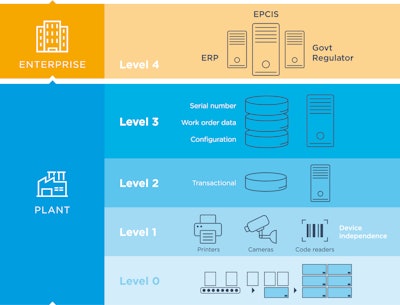
To be considered truly comprehensive, serialization solutions need to support the requirements of all levels of technology. According to ISA95, there are five levels of track, trace, and serialization technology—Levels 0 to 4—and the most comprehensive solutions, which consist of both hardware and software, connect all levels.
The promise of a Level 4 system
While Levels 0 to 3 are connected to plant/site operations, Level 4 is referred to as the “enterprise level,” where cloud-based solutions provide a portal into the overall supply chain. From manufacturing to dispensation, all stakeholders benefit from sharing information at various points in a product’s lifespan. The core principles of a Level 4 system position it to deliver on its promise to enable data sharing up, down, and across the ecosystem of trading partners, working together to bring products to market.
The ability to share information securely and near instantaneously, coupled with serialization and the unique identification of each product, is what makes true traceability possible. A Level 4 system has the potential to usher in a new era of supply chain efficiency, transparency and compliance. We have entered the era of the digital supply chain—the transformation it will drive has just begun.
Elements of a Level 4 system
A best-in-class Level 4 system uses multiple standard data exchange protocols, exchange patterns, data security and data integrity techniques.
User interface (UI): The UI must be clean, crisp and modern, and responsive to the existing system’s dimensions. It should be 100% responsive for mobile phone usage, and “scale up” for larger monitor displays.
Centralized location: Centralization and secure connectivity are important at Level 4, as it is common for various user and system roles within an organization to require access to various functional areas or APIs. For example, warehouse and distribution center workers need to perform business processes on data collected at the packaging line (Level 2) and aggregated by a site-level system (Level 3). Before these workers can do their jobs, Level 3 must send relevant data to Level 4. Without a proper Level 4, warehouse workers and distribution center workers would be operating on different networks and unable to share important data. The Level 4 system must be centrally located and accessible by authenticated and authorized users and systems.
Security: Identity Access Management (IAM) is critical, as not all actors—whether human or electronic—will have the same access to Level 4 functionality and API operations. A secure Level 4 will use Multifactor Authentication (MFA), Certificates, API Keys, and HTTPS, at a minimum.
Electronic signature: FDA Regulation CFR 21 Part 11 clearly states that all changes within systems dealing with pharmaceutical operations must record electronic signatures for any changes made to data.
Auditing: It’s critical that all Level 4 actions taken by any actor be audited. Audits include actor identifiers, dates and times, underlying objects changed, and values before and after change.
Centralized logging: This describes the ability to collect and centralize logs from site-wide Level 3 systems, as well as the Level 4 system, and to provide insights into that data. Centralized logging provides valuable, system-wide usability metrics and faster problem resolution.
Message intermediation: Level 4 is where message interoperability between two or more systems occurs, transforming AJAX into XML, XML into CSV, or one XML format into another XML format, before data is moved to another system.
Level 4 systems help ensure global compliance
Since different countries have different requirements, flexibility must be factored into any system. Some governments require systems to send properly formatted data directly to a central system; others take a more manual approach, such as email of (S)FTP. Regardless, the Level 4 system must be able to accommodate and record the workflow.
The ability to inspect the aggregation of a Batch/Lot or Object Identifier, such as an SSCC or SGTIN, is vital, and entails a variety of identifiers, including:
a. Disposition
b. Packaging level (pallet, case, item, etc.)
c. Genealogy (children, parent, grandparent, etc.)
d. Last known location (GLN or LAT. LONG. Pinned on a Mapping API)
e. Current location (GLN or LAT. LONG. Pinned on a Mapping API)
Serial numbers management: Although Level 4 isn’t the primary stage for recording product serial numbers, some degree of serial number management is useful, depending on the recording system being utilized.
For example, if a manufacturer uses our Adents Supervisor, this system would be its “system of record” for serialization numbers. It’s useful for the Level 4 solution to review serial number ranges in order to inspect and audit Supervisor’s issuance of serial numbers, and to have the Level 4 system configure serial numbers and communicate new and updated information back to Level 3.
Business processes: A Level 4 system should be able to execute business processes, defined as any problems that a customer must solve in order to be compliant with government regulations and/or their own standard operating procedures. Examples include packing, shipping, disaggregation, global compliance reporting, printing, and sending/receiving files or data to and from other systems.
Label printing: In a warehouse or distribution center, it’s not uncommon for labels to get destroyed, or for rework to occur requiring additional label printing. A Level 4 solution that assures compliance must accommodate instances of both reprinting and, should a revised serial number be required, new label printing.
Reporting: Reports are always a requirement in any enterprise-level software system, and can be described as either industry reports or customer-driven reports.
The Cloud fit
There are advantages to deploying any modern system in the Cloud, including high availability, unlimited scalability, durable storage, database management (including identity access segregation), and metrics mining (i.e. big data) capabilities. While these capabilities are desirable in any enterprise application, the Cloud makes them a reality with less effort, and at tremendous cost savings, when compared to onsite physical hardware alternatives.
Data integrity, security, and resilience benefit greatly from built-in features of Cloud infrastructure. Data centers thousands of miles apart can be set up in minutes, making data available to dependent locations while protecting it from loss. Backing up, restoring, and ensuring consistency among data centers is clean and concise.
Another benefit of the Cloud is the maturation of DevOps. Building, testing, and deploying software on the Cloud can follow predictable and repeatable processes that were not easily achievable just a few years ago.
New digital paradigm
At its core, the purpose of the supply chain is to bring a product from manufacturer to consumer, at least in the physical world. In the digital world, manufacturers, products and consumers are represented by a broader, information-driven definition. A manufacturer carries information about the components, partners and equipment used to make a product; this product has a digital image of itself carrying its composition, characteristics and usage information (i.e. manual); a consumer has a profile with context, history, behavior, etc. In the digital realm, the supply chain collapses and is freed from the barriers imposed by the physical world.
Imagine this: A consumer has the potential to access full information, including the origin and authenticity of a product from his or her smartphone; a manufacturer to manage production capacity in real time and receive instant feedback from the consumer; and products to become more personalized, more engaging and closer to the consumer's needs and expectations.
Then imagine that scenario being put into place in conjunction with the introduction of emerging technologies, such as Blockchain, which brings new ways to secure and protect the integrity of each actor and transaction within the digital supply chain. Clearly, Level 4 systems are at the heart of a new and exciting digital paradigm for both manufacturers and consumers.
Editors note: Christophe Devins is the CEO and Co-founder of Adents. Chuck Sailer is a Serialization Solutions Expert for Adents, which operates globally, with offices in Europe and in the U.S., with a worldwide network of solution partners.