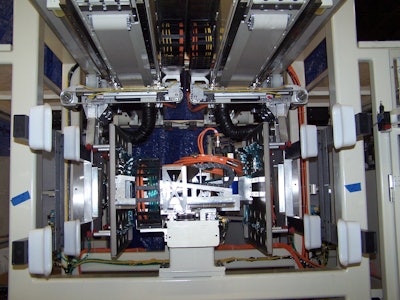
CBW Automation debuts its SSE robot, the CBW, a high-flow vacuum system that helps customers achieve a fast transfer of parts from mold to automation for downstream operations.With the high-flow vacuum system, the SSE robot achieves an intrusion time of less than 0.5 sec. Additionally the SSE can recognize that the vacuum has been achieved in as little as 60 msec.
While CBW is known for its high-speed retrieval systems, the newest design of the SSE robot incorporates a strip stroke. Combining the high-flow vacuum and the strip stroke feature provides the following benefits:
• Allows for more complex mold and part geometries
• Allows for undercuts to be stripped quickly
• Allows for the robot to follow the motion of the mold for unscrewing or more complex ejection of parts
SSE robot comes fully tested and has a solid steel frame construction and PC-based control platform, allowing remote diagnostics and troubleshooting. Additionally, the SSE robot is backed by a one-year parts and service warranty.





















