Worldwide, the market for prefilled syringes “is surging [at] a significant rate, due to an increasing geriatric population, increasing demand for vaccines, increasing prevalence of chronic and lifestyle diseases, and technological advancement in prefilled syringes.”
That’s according to ResearchAndMarkets, which defines prefilled syringes as “a single use and disposable format packet of parenteral drugs.” The market research store says the global prefilled syringe market value in 2014 was $3,905.1 million. It predicted the market to rise at a Compound Annual Growth Rate of 12.9% through 2020 in a recent market report.
One company that provides an example of this growth is Fresenius Kabi, a global health care company that specializes in medicines and technologies for infusion, transfusion and clinical nutrition. The company’s products and services are used to help care for critically and chronically ill patients.
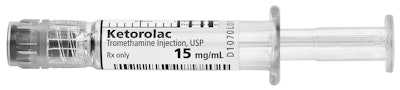
In late September, the Lake Zurich, IL-based firm announced the U.S. launch of Ketorolac Tromethamine Injection (15 mg/mL) in the company’s Simplist™ ready-to-administer prefilled glass syringe. Ketorolac Tromethamine is a nonsteroidal anti-inflammatory drug (NSAID) indicated for the short-term (up to five days in adults) management of moderately severe acute pain that requires analgesia at the opioid level, usually in a postoperative setting. It serves as a generic alternative to Toradol®.
The ready-to-administer syringes of Ketorolac Tromethamine Injection are available in 15mg/mL, 30 mg/mL and 60 mg/2mL strengths. Fresenius Kabi manufactures both the actual product and the syringes, which it says is not unusual for the fully integrated pharmaceutical manufacturer and distributor.
The combined vial/syringe package consists of the glass vial, a stopper, ferrel, latex-free ring, the flip top and the label—that includes FDA-required details such as product name and dosage—that the company applies. The syringe includes a glass barrel, a plastic stopper, a luer-lock adapter and a latex-free cap. Fresenius handles filling and labeling functions.
“With this product addition, Ketorolac prefilled syringes are now available in three presentations, providing more ready-to-administer options to our customers,” says John Ducker, President and CEO of Fresenius Kabi USA. “Similar to all Simplist syringes, this presentation of Ketorolac offers clear and consistent labeling, no assembly or point-of-care preparation and a 24-month shelf life.”
Prefilled injectable medicines are designed to help improve patient care and safety by decreasing the number of steps in the traditional vial and syringe injection sequence, reducing the potential risk of medication error.