By February 2019, pharmaceutical companies that manufacture or sell their products in Europe must have implemented the regulations specified in the EU Falsification Directive (FMD). This includes the traceability (track and trace) of prescription drugs back to the manufacturer.
STADA Arzneimittel AG responded to these requirements early on and initiated the appropriate measures. For the implementation of the serialization requirements according to EU-FMD, the manufacturer of generics and OTC brand products relies on Laetus GmbH as its partner. The project covers a total of 30lines at the sites in Germany, Serbia, Montenegro, UK and Russia.
In choosing Laetus, STADA Arzneimittel AG is placing its trust in a reliable partner with a proven ability to implement serialization projects of this scale and deliver success—backed up by more than 40 years’ experience of in-line quality control and hundreds of successful track-and-trace installations.
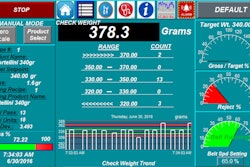
In addition to Secure Track & Trace software (S-TTS), Laetus also offers some 20 MV-70-F pack handling systems with integrated weighing and manipulation protection functions (tamper-evident labeling). This machine is suited to integration into existing packaging lines thanks to its compact dimensions.
STADA Arzneimittel AG is a publicly-listed company with headquarters in Bad Vilbel, Germany. STADA consistently focuses on a multi-pillar strategy of generics and branded products (OTC) with an increasingly international market orientation. The Group is the only independent generics producer in Germany.
Founded in 1974, Laetus produces inline quality inspection systems with thousands of successful installations of its ARGUS, POLYPHEM, and INSPECT solutions across identification, fill inspection, and other quality control applications.With its Secure Track and Trace Solution (S-TTS), Laetus is also a leader in developing robust supply chain solutions for the pharmaceutical, medical, cosmetics and FMCG industries enabling the requirements of all regulations regarding anti-counterfeiting to be fulfilled.