From more traceable pharmaceuticals to better batches of Teflon to recipe-managed clean-in-place systems, the ISA-88 Batch Control standard seems to be the gift that keeps on giving. Its clear definitions, models and methodologies for designing and operating control systems go beyond simple batch processing applications to enable flexible manufacturing of all types. The standard is delivering benefits years after the first of its five parts—“Models and Terminology”— was approved in 1995.
The ANSI/ISA-88 standard (also known as IEC 61512-3 or S88, for short) enables a universal model for batch control that eliminates the difficulty of communicating requirements and integrating systems from different vendors. As such, it is facilitating the development of new “recipe-based” systems in other industries.
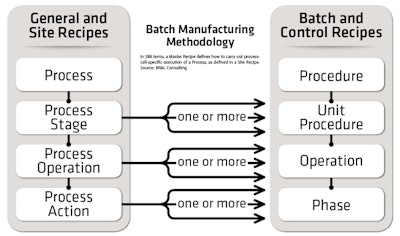
“S88 applies to flexible manufacturing operations of all types, including batch. Its key concept is separating product information (recipes) from equipment capability, and it applies to any level of automation and any type of automation equipment,” explains Dennis Brandl, chief consultant of BR&L Consulting (www.brlconsulting.com).
S88 general and site recipes describe only processing requirements, not the specific target equipment or controller hardware. The S88 model does, however, describe the recommended practice for structuring control and control logic to achieve the required flexibility, says Brandl.
>> ISA-88: Recipe Changes Kept Separate From Control Changes. Click here for more information.
Brandl is a former chairman of the ISA SP88 Batch System control standard, a member of ISA’s SP95 Enterprise/Control System Integration committee, and a U.S. expert on batch control to the International Electrotechnical Commission (IEC). He says S88 represented a “major transformation” when it was introduced, and its next innovations will come from its integration with ISA-95 to enable recipe definitions to be exchanged across the enterprise. But system integrators and their end-user companies are benefitting today from systems built on the standard.
DuPont improves Teflon production
Three years ago, management at DuPont (www.dupont.com) decided that the company’s Washington Works Teflon production operation in Wood County, W.Va., needed to improve its productivity and reduce its operational costs. The Washington Works facility was producing more than 700 million pounds of polymer and thermoplastic products annually, but its 25-year-old legacy batch system was experiencing end-of-life issues and costly maintenance spare-parts-availability concerns. It also didn’t comply with company standards. So management sought to migrate to a modern control system that included a batch system aligned with S88 guidelines.
David Niermeyer, senior process controls engineer for DuPont Washington Works, says DuPont decided to replace the plant’s outdated controls with Honeywell’s (www.honeywellprocess.com) Experion Process Knowledge System (PKS) Release R400 running the Experion Batch Manager application.
“Because this solution is built on S88 principles and hierarchies, it provided the redundancy features demanded by DuPont’s batch processes, allowing plant personnel to execute batches using multiple batch units from the procedure level downwards,” says Chris Morse, product manager for Experion PKS.
Washington Works’ legacy system was not S88 structured, and its software ran on non-redundant servers that were susceptible to outages, opening the possibility to having to scrap a batch. The modularity of modern S88-based systems allows recipe logic to run on whatever hardware platform the user finds appropriate, from small controllers to large distributed server architectures.
Morse says Experion Batch Manager is able to execute all four levels of the S88 model in the Experion C300 controller. Hardware redundancy increases the availability of the platform, he says, and “since the batch executes in a controller environment, the sequence execution cycle time is configurable up to 50 ms.”
When you have a split architecture (some code running in the controller and some on the server system), handshaking between controller and server introduces dead time, adds Morse. “If you take the functionality into the controller, that can reduce the cycle times for a batch significantly. You can get 2-3 percent more from a platform,” he says. That flexibility regarding software execution—without any rewriting of code—is what S88 makes possible. It also enabled development of an advanced simulation solution, and helped DuPont operators get up to speed on the new systems quickly.
“The way the operators thought and the way the legacy system operated were quite different from the modern system,” says Morse. “A sister plant in China had already completed a similar control system project, and lessons from that effort provided valuable insights. Because the legacy system was not S88-based, there was a degree of training there regarding new displays and new tools, but the [S88] structure made all the little nuances easier to understand.”
The new plant automation and batch management capabilities already have improved both uptime and yields for DuPont, and they are expected to reduce cycle time too. Additionally, the plant has shortened the period between batches, which should improve productivity over the long term, says Morse. Work is still in progress, slated for completion over four phases spanning 2011-2014.
Large multinationals like DuPont will often specify ISA-88 compatibility because they want to ensure all their plants can flexibly change products and recipes, and start up new operations quickly. But anyone who buys software from the major vendors benefits from S88.
Baxter improves traceability
The ISA-88 standard drove automation software vendors to adopt structured and hierarchical models into their systems at a time when PC-based systems and human machine interfaces (HMIs) were just being introduced. “The rise of PC technology in the industry allowed for more graphical modeling tools, such as the structured flow charts for recipe procedures and graphical configuration methodologies,” says Michael Schwartz, a product marketing manager for Invensys Wonderware (www.wonderware.com) InBatch software. “I would be surprised if any end user would accept a batch management system without implementation of the ISA-88 standard today.”
Use of standard software and defined interfaces to control systems, and recipe execution in separate phases all help to “reduce the cost and efforts to qualify and validate a batch management system in regulated industries,” says Schwartz. “This is an indirect effect of transparent, structured ISA-88 recipe descriptions, and matching structures in control systems for recipe execution,” he says.
Schwartz cites the implementation of a new nutriments production line for Baxter S.A. The new line, composed of six processes for preparing the various nutriments and filling intravenous storage bags, had to double existing production capacity while significantly reducing throughput time, integrate each of the facilities subsystems to create a fully automated process, and assist Baxter in achieving FDA 21 CFR Part 11 compliance.
To achieve these goals, Baxter used a variety of Wonderware software from Invensys Operations Management (www.iom.invensys.com), including Wonderware InBatch for batch execution and genealogy reporting; Wonderware InTouch HMI for process visualization; and Wonderware System Platform to support all SCADA, supervisory HMI and MES applications. These Invensys systems have ISA-88 models and methodologies within them, and information is synchronized within ArchestrA, the company’s system architecture that is itself based on a distributed, object-based design.
System integrator BiiON, which specializes in the pharmaceutical and biotechnology industries, worked with Invensys and Baxter to ensure successful implementation of the new line and of Wonderware Historian and eSignature management applications, used to ensure Baxter’s compliance with FDA 21 CFR Part 11 regulations. “By establishing the automation framework, Wonderware software solutions enabled our project teams to focus on productivity targets,” says Serge Bassem, BiiON CEO.
At Baxter, a secure web server hosts the user interface application for recipe management and configurable parameters, as well as graph, audit trail and report generation systems. It also centrally houses a custom set of S88-compliant objects that can be reused for future projects. BiiON engineers created roughly 35 reusable generic objects for functions such as valves, sensors, weighing equipment and variable-speed drives, says Bassem.
“Each functional object is associated with a PLC and SCADA software code. These functional objects are linked to the processes in the system so that any changes made to the generic functional object are immediately replicated, with the ability to be linked to all instances in the system. The engineer maintains control of whether he wants to deploy modifications on all or part of the instances of the relevant object,” Bassem explains. This allows the engineering staff to validate modifications being implemented before making a global deployment.
“The Wonderware System Platform enabled us to simulate equipment behavior to encapsulate advanced functions that would enable Baxter to manage all system interactions on the production line. This allowed us to view the operation of equipment to ensure all functions worked smoothly together,” Bassem adds.
Bassem says the scalability features of the software also were “a very important factor in Baxter’s decision because the automation system would be evolving over time.”
The transfer of material is not part of the S88 model, but Schwartz says the Wonderware batch system “comes with an extended ISA-88-compliant recipe model that adds connections to the equipment model to enable transfer phases as part of the recipe, in addition to the ISA-88 process equipment and process phases. The extension simplifies recipe modeling and provides more effective equipment arbitration for concurrent batch execution on shared equipment. In combination with a materials and inventory model, this enables automatic full materials traceability and product genealogy.”
>> Click here to read how the revamping of an industrial kitchen became a S88-based configuration tool.
Such traceability of materials throughout the enterprise is also the goal of work being done by the World Batch Forum, now a part of MESA International (www.mesa.org), and others who are extending and combining the structured development methods embodied in the ISA-88 and ISA-95 standards.
In the meantime, says John Parraga, global process technical consultant for Rockwell Automation’s (www.rockwellautomation.com) Batch Process business, “The procedural side of S88 may be capturing the best practices from the quality department, the engineering side, and the operator point of view. It’s a summary, a bouillon of everyone’s procedural needs. S88 is really capturing all the best practices of all the different players in this procedural ecosystem."