In the realm of pharmaceuticals, there are some concerning statistics according to the Pharmaceutical Research and Manufacturers of America. One in ten medicines worldwide are presumed to be counterfeit. In addition, 95% of internet drug outlets have been found to be out of compliance with federal and state pharmacy laws and practice standards. During one week alone in March of 2020, over 48,000 packages containing counterfeit medicines were seized by Interpol.
In serialization, systems assign a unique serial number linked to information about the product origin, batch number, and expiration date to each saleable unit of each prescription drug product. According to a new report by PMMI Business Intelligence, “Pharmaceutical & Medical Devices | Trends & Opportunities in Packaging Operations,” regulations vary worldwide.
In the United States, the federal Drug Supply Chain Security Act (DSCSA), enacted in 2013, establishes a system to track and trace prescription drugs throughout the U.S. supply chain. The key purposes of DSCSA are to verify the legitimacy of the drug product identifier down to the package level; to make drug recalls more efficient; and, to enhance detection of illegitimate products in the drug supply chain.
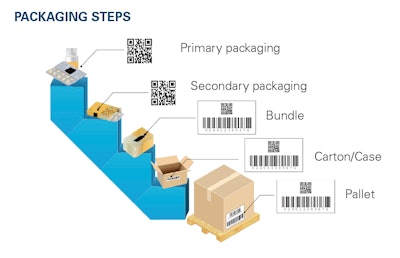
The goal of DSCSA is unit-level traceability by 2023, including aggregation, or being able to trace a single unit to the larger bundle, case or pallet that it came from. 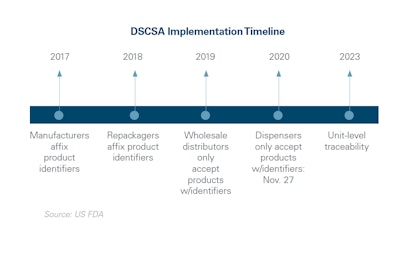
Implementing serialization technology creates challenges beyond compliance at all stages of the packaging process due to the numerous steps involved.