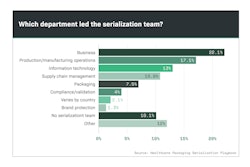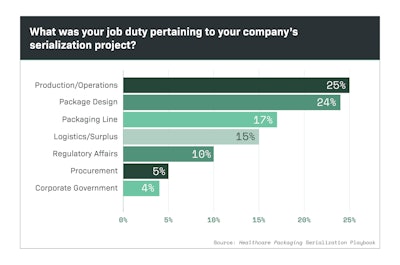
Part Two of a Special Report on serialization demonstrates the wide range of job duties tasked with the responsibility of meeting this mandate.
With serialization serving as a regulatory mandate, one might argue that meeting those deadlines would fall under a company’s regulatory umbrella. Yet that wasn’t necessarily the case, according to Healthcare Packaging’s Serialization Playbook survey respondents.
Beginning in 2012 and running through 2018, the Playbook was downloaded more than 4,700 times. Each person answered a series of questions that revealed details such as where they worked, their role in serialization, as well as key metrics such as how many lines they were responsible for serializing and where they were located.