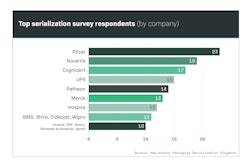
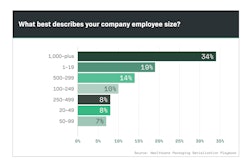
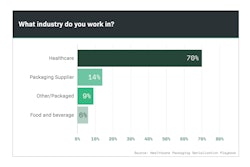
Beginning in 2012 and running through 2018, Healthcare Packaging’s exclusive Serialization Playbook Survey was downloaded more than 4,700 times. Respondents from the U.S. as well as countries around the world represented multiple industries—not only the life sciences, but some from outside. Respondents seeking info on serialization standards, etc., ranged from end-user manufacturers to suppliers and consultants. Each person downloading the 150-page playbook answered a series of questions to tell Healthcare Packaging editors who they were, where they worked, their role in serialization, and key metrics like how many lines they were responsible for serializing and where they were located.
The playbook responses in the following nine articles and multiple charts help paint a picture of Serialization in the Rearview Mirror for the pharma plant floor.