This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
Pre-qualified for last-mile pharmaceutical delivery of refrigerated products, the TempAid thermal-insulated mailer with integrated PCM refrigerants allows specialty pharma and mail-order drug organizations to transport individual doses of medications that must remain in a cool, refrigerated environment at a stable temperature within a sleek envelope form factor.
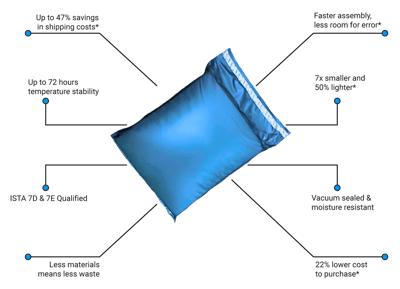
It is tested in the TempAid ISTA Certified Thermal Laboratory for up to 72 hr of temperature stability using the ISTA 7D and 7E standard.
The mailer:
- Saves shipping costs by up to 77% vs. standard shipping solutions
- Requires no assembly, reducing the risk of pack-out failure
- Costs up to 22% less than standard shipping solutions
- Is seven times smaller and 50% lighter than standard shipping solutions
- Is vacuum packed for shipping to reduce shelf storage space
Companies in this product






















