This content was written and submitted by the supplier. It has only been modified to comply with this publication’s space and style.
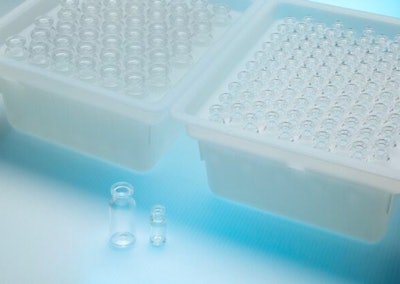
West Pharmaceutical Services, Inc., a global provider of solutions for injectable drug administration, unveiled its new Daikyo Crystal Zenith (CZ) Nested Vials in Tub.
Designed to meet the exterior dimensions of glass standard ISO-8362-1, CZ nested vials are a demonstrated containment solution for cell and gene therapies, radiopharmaceuticals, and sensitive molecules. CZ's performance attributes, such as maintaining container closure integrity down to cryogenic temperatures, superior functional recovery of viral vectors, low particulate specification (2% USP <788>), break resistance, and low risk of chemical interaction, make it suited for advanced therapies. CZ vials have demonstrated effectiveness in safely storing gene therapy drugs and meeting primary packaging demands from preclinical to commercial phases.
Product specifications
- CZ nested vials are available in 2mL and 10mL, and with ISO 8362-1 exterior dimensions.
- These vials offer flexible closure solutions with crimped closures using NovaPure® stoppers or PLASCAP® press-fit closures with Daikyo stoppers to meet manual, semi-automated or automated capping requirements.
- The ready-to-use nested vials are first layered with a Tyvek® slip sheet, heat sealed with a breathable Tyvek® lid, double bagged and sterilized.
- The rigid structure and overall design of the vial nest tub protects the vials during transit and promotes machinability.





















