ISSUE: Phoenix Import & Distribution LLC and FDA notified the public of a recall for Pentrexyl Forte Natural because the packaging is believed to be misleading, causing it to be confused with an antibiotic. Pentrexyl Forte Natural is a Dietary Supplement only and does not contain antibiotics. Use of the product by ill individuals could delay treatment for serious illnesses.
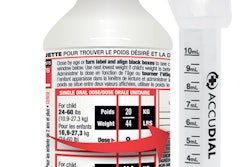
BACKGROUND: The product is packaged in a green and white box, containing 30 red and white capsules. The product was sold through retail stores in Texas.
RECOMMENDATION: Consumers who have purchased the product can return it to the place where it was purchased.
Healthcare professionals and patients are encouraged to report adverse events, side effects, or product quality problems related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
•Complete and submit the report Online
•Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
FDA MedWatch - Pentrexyl Forte Natural: Recall - Misleading Packaging
Phoenix Import & Distribution announces recall of Pentrexyl Forte Natural dietary supplement.
May 25, 2011
Researched List: Blister Machines for Life Sciences
Need a blister machine for life sciences packaging? Our curated list features companies serving pharmaceutical, medical device, nutraceutical, and cosmetic industries. Download to access company names, locations, machine specifications, descriptions, and more.
Download Now
Start 2026 with Smarter Packaging for Life Sciences at PACK EXPO East!
Be the first to find what’s next in packaging for life sciences at PACK EXPO East. See solutions from 500 exhibitors, gain inspiration in free educational sessions and uncover new ideas for your industry and beyond—all in one trip to Philadelphia.
REGISTER NOW & SAVE
Downloads