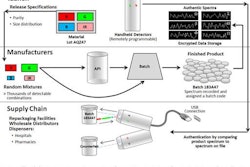
The FDA is proposing revisions to its annual reporting requirements for drug sponsors of all antimicrobials sold, or distributed for use, in food-producing animals in order to obtain estimates of sales by major food-producing species.
The additional data would improve understanding about how antimicrobials are sold or distributed for use in major food-producing species and help the FDA further target its efforts to ensure judicious use of medically important antimicrobials.
The proposed rule also includes a provision to improve the timeliness of the report by requiring the FDA publish its annual summary report of antimicrobial sales and distribution information by Dec. 31 of the following year.
Current regulatory authority limits the data collection that FDA can mandate to antimicrobial sales and distribution information.