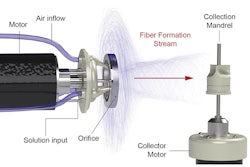
A recent New York Times article said the FDA plans to regulate the prenatal testing market amid concerns about false positive results and potentially misleading marketing. The regulations would target noninvasive prenatal tests that use a small blood sample to screen for genetic abnormalities during the first trimester. While these tests are accurate for common genetic disorders like Down syndrome, they often provide incorrect positive results for rare abnormalities.
The misleading language used by companies to describe the tests can lead to significant distress for parents and even result in the termination of pregnancies based on false positives. The FDA's concerns extend beyond prenatal screenings and include tests for heart disease and autism that may lead to improper treatments. The agency aims to address these issues through new regulations and a pilot program for laboratory-developed tests used in cancer drug trials. However, legal challenges from test manufacturers who dispute the FDA's authority to regulate such products are expected.