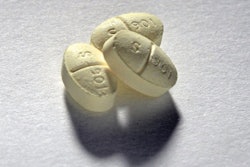
Earlier this summer, Pfizer halted global distribution of its smoking cessation drug, Chantix. A few weeks later, the company recalled over a dozen lots of the drug. Now, according to a Fierce Pharma article, the drugmaker is recalling all 0.5 mg and 1 mg lots across the country. The reason is the the drug was found to contain unacceptable levels of a specific nitrosamine, which can cause cancer over time with exposure to high levels. The drug was approved by the FDA 15 years ago, and saw nearly a billion in sales last year.
Pfizer Recalls All Chantix Nationwide
The recall is a precautionary measure taken after the medicine was found to contain a cancer-causing impurity.
Sep 23, 2021
Researched List: Blister Machines for Life Sciences
Need a blister machine for life sciences packaging? Our curated list features companies serving pharmaceutical, medical device, nutraceutical, and cosmetic industries. Download to access company names, locations, machine specifications, descriptions, and more.
Download Now
List: Digitalization Companies From PACK EXPO
Looking for CPG-focused digital transformation solutions? Download our editor-curated list from PACK EXPO featuring top companies offering warehouse management, ERP, digital twin, and MES software with supply chain visibility and analytics capabilities—all tailored specifically for CPG operations.
Download Now
Downloads