Key takeaways:
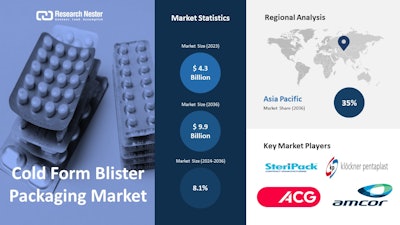
· The global cold form blister packaging market size is predicted to surpass more than $9.9 billion by 2036
· The PVC segment is expected to gain a significant market share during the forecast period
· There is a need for sustainable cold-form blister packaging solutions built with recyclable PET materials
According to recent study by Research Nester, the global cold form blister packaging market size is predicted to surpass more than $9.9 billion by 2036 and is projected to expand at a CAGR of 8.1% between 2024 and 2036 due to the growing investment in the healthcare sector.
Growing Demand in the Pharmaceutical Industry Global Market Share of Cold Form Blister Packaging
The expanding demand for cold-form blister packaging is driven by its remarkable qualities, including effortless sealing and providing a robust barrier against oxygen, water, light, and moisture. Cold-form blister packaging involves the use of specialized cold-forming foil, which eliminates the need for heat during the packaging process. This technique is primarily used for packaging pharmaceuticals such as tablets and capsules, providing a secure and protective enclosure for these sensitive medical products.
Therefore, the surge in the demand for blisters and the maximizing research and development activities in the pharmaceutical industry are boosting the market growth. Also, the pharmaceutical sector has witnessed sustained growth over the decades, due to the geriatric population, growing healthcare awareness, and the demand for numerous medications. According to WHO, one in six individuals on the globe will be 60 years of age or older by 2030.
Some of the major growth factors and challenges that are associated with the growth of the global cold form blisters market are:
Growth Drivers:
· Surge in the adoption of cold form aluminum blister packs
· Exceptional features than the alternative packaging types
Challenges:
The most popular type of blister pack is thermoformed, which employs clear PVC material provides a variety of seal options, and safeguards the goods during travel as it is strong, resilient, and impervious to tampering. Besides this, heat seal blister cards, a form of blister packing produced by applying heat, are the perfect packaging option for pharmacies to shield food items and medications from impurities, moisture, and air. All these factors may limit the adoption of cold form blister packaging.
Moreover, the fluctuating prices of raw materials and a lack of advanced infrastructure in emerging nations are some other challenges that may hamper the growth of the cold form blister packaging market.
By material, the global cold form blisters market is segmented into aluminum, PVC, and PET. The PVC segment is expected to gain a significant market share during the forecast period. The main advantage of PVC is its affordability. PVDC, or polyvinylidene chloride, is mixed with PVC sheets to improve the packaging's barrier properties. This provides outstanding protection against moisture and oxygen.
By region, the Europe cold form blister packaging market is expected to garner notable market revenue. The region's market is expanding due to the growing emphasis on child-resistant packaging and the rising need for unit-dose packaging provided by cold-form blister packaging solutions. Furthermore, the need for sustainable cold-form blister packaging solutions built with recyclable PET materials that support the region's sustainability goals is being driven by a shift in consumer preference for eco-friendly packaging options in the healthcare sector.
Top companies include in the global cold form blister packaging market are Amcor plc, Constantia Flexibles, Sonoco Products Company, WINPAK LTD, Bilcare Limited, Honeywell Internatonal Inc., TekniPlex, Liveo Research AG, R-Pharm Germany GmbH, Wasdell Group, UFlex Limited, and others.
Source: www.researchnester.com/reports/cold-form-blister-packaging-market/6334