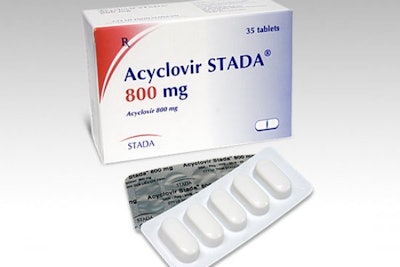
A recent FDA MedWatch article reported an unfortunate recall in which a packaging error led to the wrong drug being mixed into blister packs. On February 13th Apace Packaging voluntarily recalled one lot of Acyclovir tablets, an antiviral drug used to treat shingles, genital herpes, and chickenpox. A missed dose of Acyclovir could lead to the reactivation of the virus being treated.
The recalled lot of 400mg tablets in UD blister cards could contain 20mg tablets of Torsemide, a prescription drug used to treat edema and hypertension. Accidental dosing of Torsemide could cause excessive urination as well as other undesirable symptoms including chest pain, diarrhea, and rectal bleeding. Apace notified distributors and customers of the packaging error via email and is arranging for the return of all recalled product.