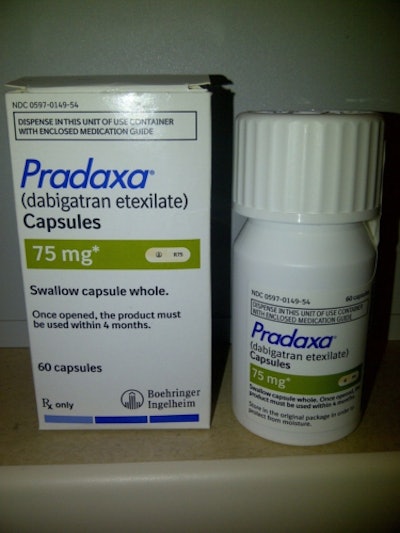
Boehringer Ingelheim Pharmaceuticals, Inc. announced Nov. 7 that it is conducting a nationwide voluntary recall of a single manufacturing lot of Pradaxa® (dabigatran etexilate mesylate), 75mg 60 US, NDC 0597- 0149-54, lot 201900, Exp. Jan. 2015. Pradaxa is indicated to reduce the risk of stroke and systemic embolism in patients with non-valvular atrial fibrillation (NVAF). The recall is limited to this one lot number.
This recall is being conducted due to a potential packaging defect on this lot that may compromise the bottle integrity. A damaged bottle could allow moisture to get into the bottle and, thus, may impair the quality of Pradaxa. As a consequence a patient may not receive a fully effective dose of Pradaxa 75mg, which would increase his or her risk of experiencing an ischemic stroke. This risk is small; however, not zero. Therefore, as a precautionary measure, Pradaxa is being recalled at the patient level.
According to the company, actions for patients with bottles of PRADAXA 75mg from lot 201900 include:
• Patients should continue to take the product as directed until they obtain replacement to assure there is no interruption of therapy.
• We believe most of the potentially affected bottles have been returned, but if a person has or receives a bottle of Pradaxa 75mg from the potentially affected lot he/she should return the potentially affected bottle to their pharmacist as soon as possible for replacement at no charge.
Information has been sent to pharmacists alerting them of the details pertaining to this recall. Pharmacists who may have dispensed Pradaxa capsules to patients from manufacturing lot 201900 are instructed to contact those patients to return the product lot back to the pharmacy.
Distributors/retailers that have not received a recall packet should contact GENCO Pharmaceutical Services, 6101 North 64th Street, Milwaukee, WI 53218.
For information regarding this recall, the company provided the following telephone numbers:
• For technical product information or to report a technical product complaint, call 1-800-542-6257, option #3.
• For information regarding the recall process, call GENCO Pharmaceutical Services at 1-877-319-8963.
Ridgefield, CT-based Boehringer Ingelheim Pharmaceuticals, Inc., is the largest U.S. subsidiary of Boehringer Ingelheim Corp., and a member of the Boehringer Ingelheim group of companies.